Copper catalyst for direct method synthesis of methylchlorosilane and preparation method thereof
A technology of methylchlorosilane and copper catalyst, which is applied in the fields of chemical instruments and methods, organic chemistry, compounds of group 4/14 elements of the periodic table, etc., can solve the problems of low catalytic activity and insufficient catalyst preparation methods, and achieve The effect of improving catalytic activity, improving mass transfer process, and low content of harmful impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
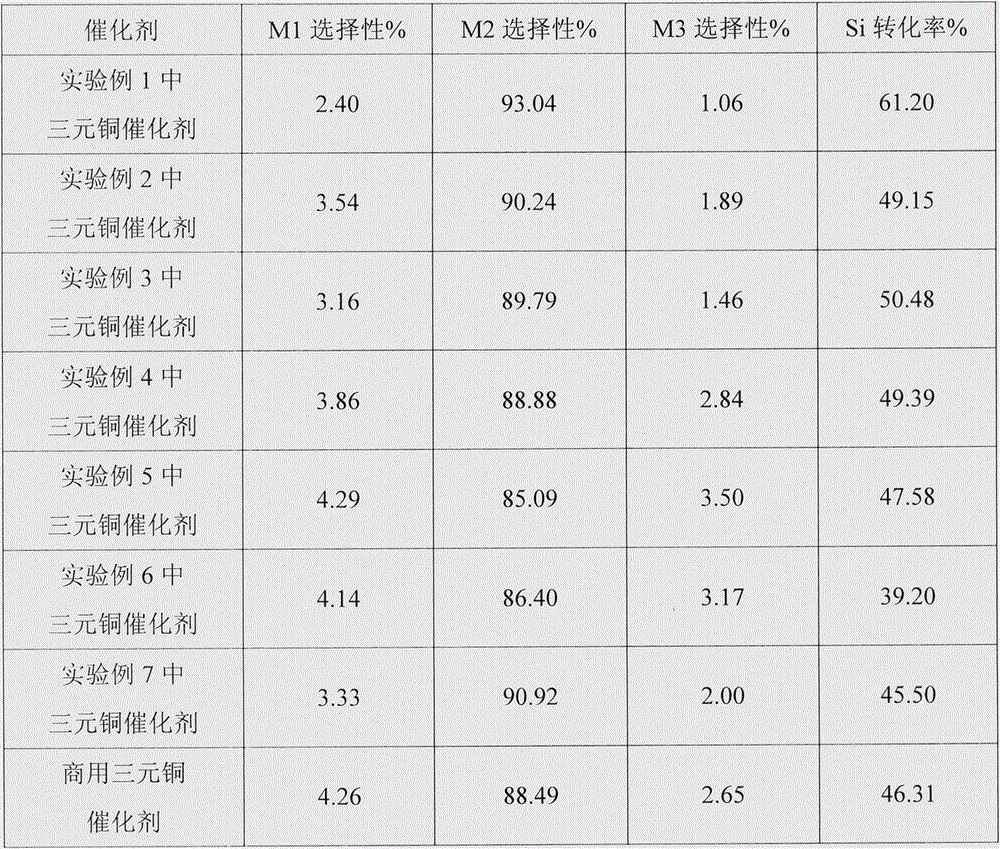
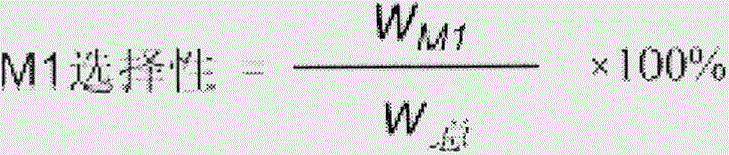
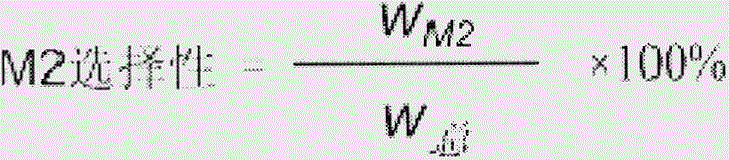
Examples
Embodiment 1
[0032] (1) Dissolve 2.00g of copper nitrate (A.R.) in a mixed solvent of 80.0ml of pure ethanol (A.R.) and 24.0ml of ethylene glycol (A.R.), mix well, then add 60ml of ammonia (25%, A.R.) and 20ml sodium hydroxide solution (1mol L -1 , A.R.), stirred to a clear solution, transferred the obtained clear solution to an autoclave for reaction, sealed and maintained at 130°C for 16 hours, and then cooled to room temperature; the reaction solution was centrifuged to obtain a precipitate, which was washed with distilled water and ethanol in turn, and then vacuum-dried , to obtain CuO metal clusters;
[0033] (2) Dehydrate and dry the CuO obtained in step (1), pass through H 2 Reduction, the reaction temperature is 150-300°C, the system pressure is from normal pressure to 0.1MPa, and Cu metal clusters are obtained;
[0034] (3) Pass the Cu obtained in step (2) into an oxygen-containing gas to oxidize, control the oxygen partial pressure to be lower than 0.02MPa, the oxidation temper...
Embodiment 2
[0037] (1) Dissolve 2.00g of copper nitrate (A.R.) in a mixed solvent of 80.0ml of pure ethanol (A.R.) and 20.0ml of ethylene glycol (A.R.), mix well, then add 60ml of ammonia (25%, A.R.) and 20ml sodium hydroxide solution (1mol L -1 , A.R.), stirred to a clear solution, transferred the obtained clear solution to an autoclave for reaction, sealed and maintained at 130°C for 16 hours, and then cooled to room temperature; the reaction solution was centrifuged to obtain a precipitate, which was washed with distilled water and ethanol in turn, and then vacuum-dried , to obtain CuO metal clusters;
[0038] (2) Dehydrate and dry the CuO obtained in step (1), pass through H 2 Reduction, the reaction temperature is 150-300°C, the system pressure is from normal pressure to 0.1MPa, and Cu metal clusters are obtained;
[0039] (3) Pass the Cu obtained in step (2) into an oxygen-containing gas to oxidize, control the oxygen partial pressure to be lower than 0.02MPa, the oxidation temper...
Embodiment 3
[0042] (1) Dissolve 4.00g of copper nitrate (A.R.) in a mixed solvent of 80.0ml of pure ethanol (A.R.) and 24.0ml of ethylene glycol (A.R.), mix well, then add 60ml of ammonia (25%, A.R.) and 20ml sodium hydroxide solution (1mol L -1 , A.R.), stirred to a clear solution, transferred the obtained clear solution to an autoclave for reaction, sealed and maintained at 130°C for 16 hours, and then cooled to room temperature; the reaction solution was centrifuged to obtain a precipitate, which was washed with distilled water and ethanol in turn, and then vacuum-dried , to obtain CuO metal clusters;
[0043] (2) Dehydrate and dry the CuO obtained in step (1), pass through H 2 Reduction, the reaction temperature is 150-300°C, the system pressure is from normal pressure to 0.1MPa, and Cu metal clusters are obtained;
[0044] (3) Pass the Cu obtained in step (2) into an oxygen-containing gas to oxidize, control the oxygen partial pressure to be lower than 0.02MPa, the oxidation temper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com