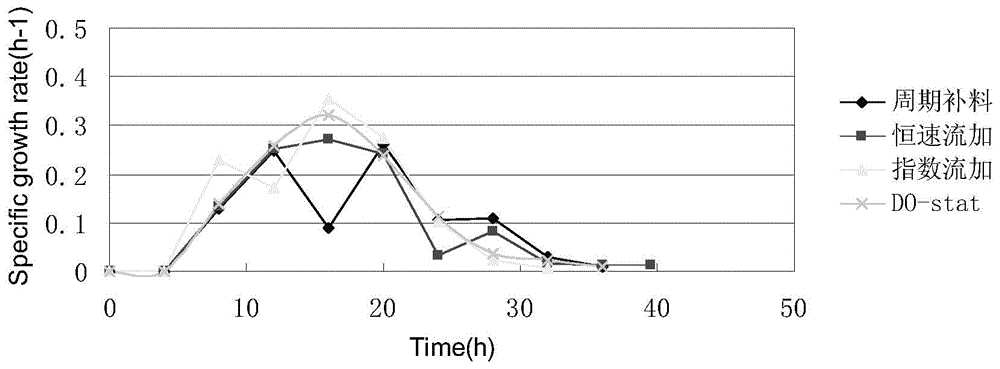
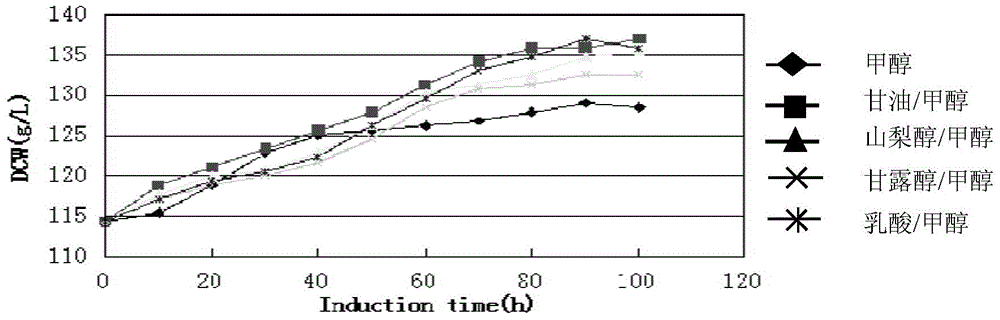
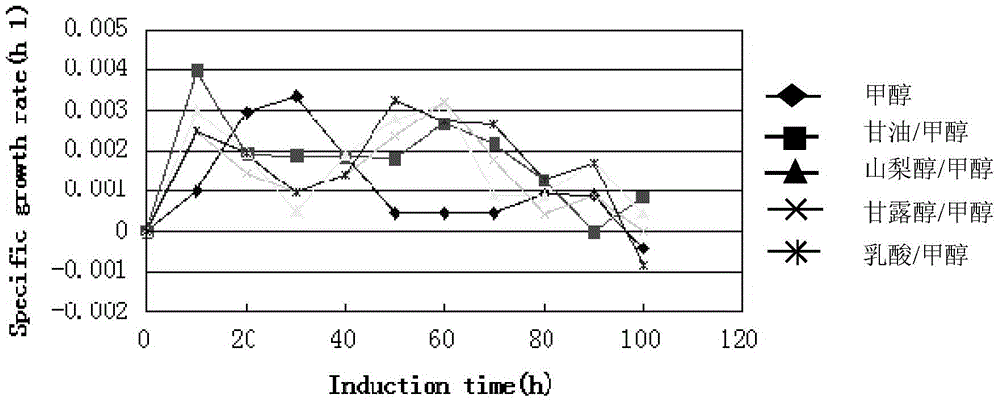
Human-derived muramidase protein fermentation method
A fermentation method and lysozyme technology, which are applied in the fields of genetic engineering and fermentation engineering, can solve the problems of high price, difficulty in ensuring no pollution from viruses and other harmful substances, and unstable product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0152] Preparation of Pichia Competent Cells: Pick a single yeast colony and inoculate it in 50mL of YPD culture medium, culture it with shaking at 30°C until OD600=1.0-2.0. Centrifuge at 5000rpm for 5min at room temperature to collect the cells. 8×10 8 The cells were resuspended in 10 mL solution A (100 mM LiCl, 10 mM DTT, 0.6 M sorbitol and 10 mM Tris-HCl, pH 7.5), and allowed to stand at room temperature for 30 min. Centrifuge at 5000rpm at 4°C for 5min to collect the precipitate, then resuspend with 1.5mL of ice-cold 1M sorbitol, centrifuge to collect the precipitate. Repeat the above steps three times. Finally, resuspend with 500 μL of ice-cold 1M sorbitol, and put it on ice for later use.
[0153] Electrotransformation of Pichia pastoris competent cells: take 80 μL (about 10 μL) of yeast competent cells resuspended in ice-cold 1M sorbitol 10 cells / mL) were mixed with 3 μg of linearized DNA, and placed on ice for 5 minutes (note: the volume of the plasmid should be co...
Embodiment 1
[0184] Example 1 Bioinformatics analysis of human c-type lysozyme The mRNA sequence encoded by Homo sapiens lysozyme (LYZ) is derived from NCBI accession number: NM_000239.2. A preferred sequence is shown in SEQ ID NO 5. For the results of the conserved site analysis between human c-type lysozyme (LYZ) and some animal c-type lysozyme / a-whey protein, see figure 2 . The isoelectric point analysis of human c-type lysozyme and some animal c-type lysozyme / a-lactalbumin is shown in Table 1.
[0185] Prediction of mature protein isoelectric point and molecular weight of H-LYZ and animal LYZ / a-whey protein with ExPasy. According to the prediction results, the PI of human c-type lysozyme protein is 9.28, the PI of a-lactalbumin is lower than that of other proteins, the PI of bovine lysozyme protein is neutral, and the rest of lysozyme are alkaline, of which turkey The PI of lysozyme protein was the highest.
[0186] Table 1 Prediction results of isoelectric point and molecular wei...
Embodiment 2
[0188] Expression of embodiment 2LYZ gene in Pichia pastoris
[0189] 2.1 Construction of expression vector pPIC9K-LYC6
[0190] Use the universal primers 5'AOX1 and 3'AOX1 of the pPIC9K vector to carry out PCR to identify positive clones. The size of the positive clones is about 890bp, and the empty vector is about 460bp. The specific results are as follows figure 1 shown.
[0191] After the pPIC9K-LYC6 (LYC6 is LYZ) recombinant plasmid was linearized and electroporated into Pichia pastoris, white single colonies could grow on the MD plate without histidine. Pick a single colony for PCR verification. Using LYC6-F and LYC6-R as primers, a specific band at about 430bp is a positive clone, indicating that the target gene has been integrated into the Pichia pastoris genome.
[0192] Phenotype screening results of transformants, almost all transformants are Mut + The phenotype is consistent with the expected result of the plasmid integration method. The clones with uniform gro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com