Quality control method for rhizoma dioscoreae nipponicae-acanthopanax root collateral-dredging capsules
A quality control method, the technology of Longjia Tongluo, applied in the field of medicine, can solve the problems of unstable yield, cumbersome operation, occurrence of side reactions, etc., and achieve the effects of good reproducibility, convenient operation and accurate results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
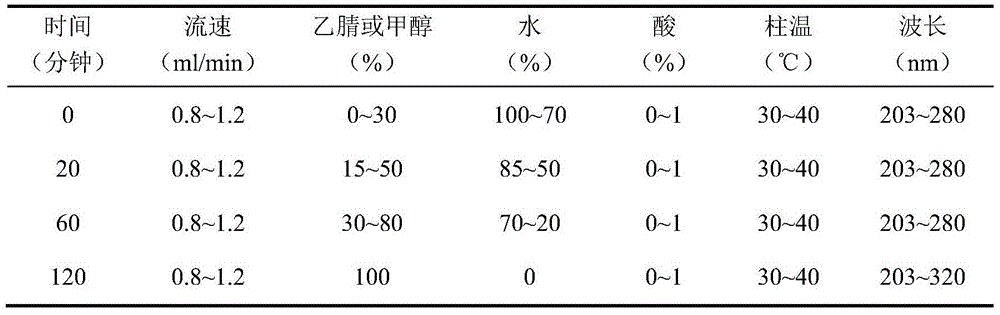
[0099] Chromatographic conditions and system suitability test: use C18 silica gel as filler; mobile phase is acetonitrile-water for gradient elution as shown in Table 14:
[0100] Table 14 gradient elution conditions
[0101]
[0102] Need testing solution preparation: get the contents of Longjiatongluo capsules, grind finely, get 2g, accurately weigh, put in a stoppered Erlenmeyer flask, add 50ml 95% ethanol, ultrasonic treatment (power 180W, frequency 40kHz) 30min, Allow to cool, weigh to make up for weight loss, filter, and take the subsequent filtrate to pass through a 0.45 μm microporous membrane as the test solution.
[0103]Preparation of reference substance solution: Accurately weigh the appropriate amount of dioscin reference substance and syringin reference substance, add methanol to make a mixed solution containing 0.6 mg of diosgenin reference substance and 10 μg of syringin per 1 ml, as a mixed reference substance solution.
[0104] Determination: Accurately d...
Embodiment 2
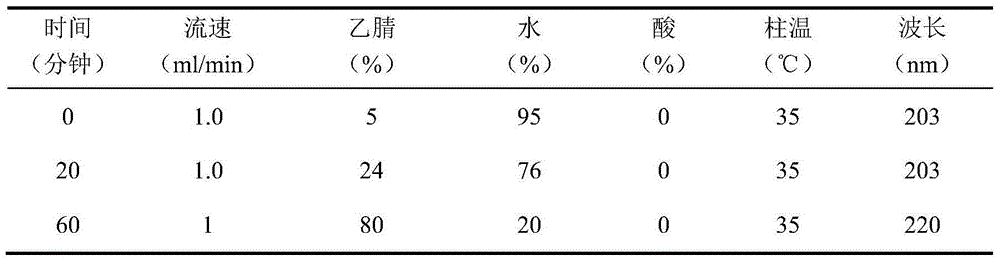
[0106] Chromatographic conditions and system suitability test: use C8 silica gel as filler; mobile phase is acetonitrile-water-formic acid for gradient elution as shown in Table 15:
[0107] Table 15 gradient elution conditions
[0108]
[0109] Preparation of the test solution: take the content of Longjiatongluo capsule, grind it finely, take 2g, accurately weigh it, put it in a stoppered Erlenmeyer flask, add 50ml of 95% ethanol, soak in cold for 120min, let it cool, and weigh to make up for the weight loss , Filtration, take the continued filtrate through 0.45μm microporous membrane, as the test solution.
[0110] Preparation of reference substance solution: Accurately weigh the appropriate amount of dioscin reference substance and syringin reference substance, add methanol to make a mixed solution containing 0.6 mg of diosgenin reference substance and 10 μg of syringin per 1 ml, as a mixed reference substance solution.
[0111] Determination: Accurately draw 10 μl of t...
Embodiment 3
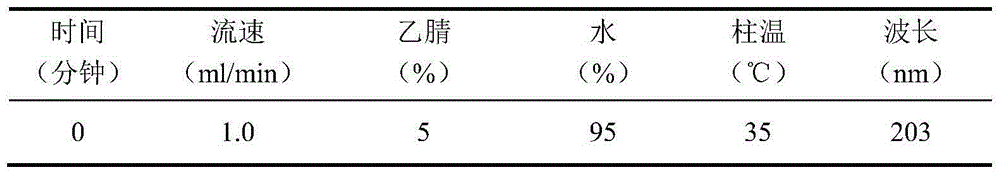
[0113] Chromatographic conditions and system suitability test: use C4 silica gel as filler; mobile phase is acetonitrile-water-formic acid for gradient elution as shown in Table 16:
[0114] Table 16 gradient elution conditions
[0115]
[0116] Preparation of the test solution: take the content of Longjiatongluo capsule, grind it finely, take 2g, accurately weigh it, put it in a stoppered Erlenmeyer flask, add 20ml of 75% methanol, reflux in a water bath for 90min, weigh it to make up for weight loss, and let it cool Filtrate, and take the continued filtrate to pass through a 0.45 μm microporous membrane as the test solution.
[0117] Preparation of reference substance solution: Accurately weigh the appropriate amount of dioscin reference substance and syringin reference substance, add methanol to make a mixed solution containing 0.6 mg of diosgenin reference substance and 10 μg of syringin per 1 ml, as a mixed reference substance solution.
[0118] Determination: Accurat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com