Method for preparing lithium titanate material
A lithium titanate, battery material technology, applied in electrical components, electrochemical generators, battery electrodes, etc., can solve the problems of poor conductivity, poor volume change cycle performance, affecting rate and cycle, etc., to solve the problem of coating Incomplete, the effect of improving rate performance, and improving conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
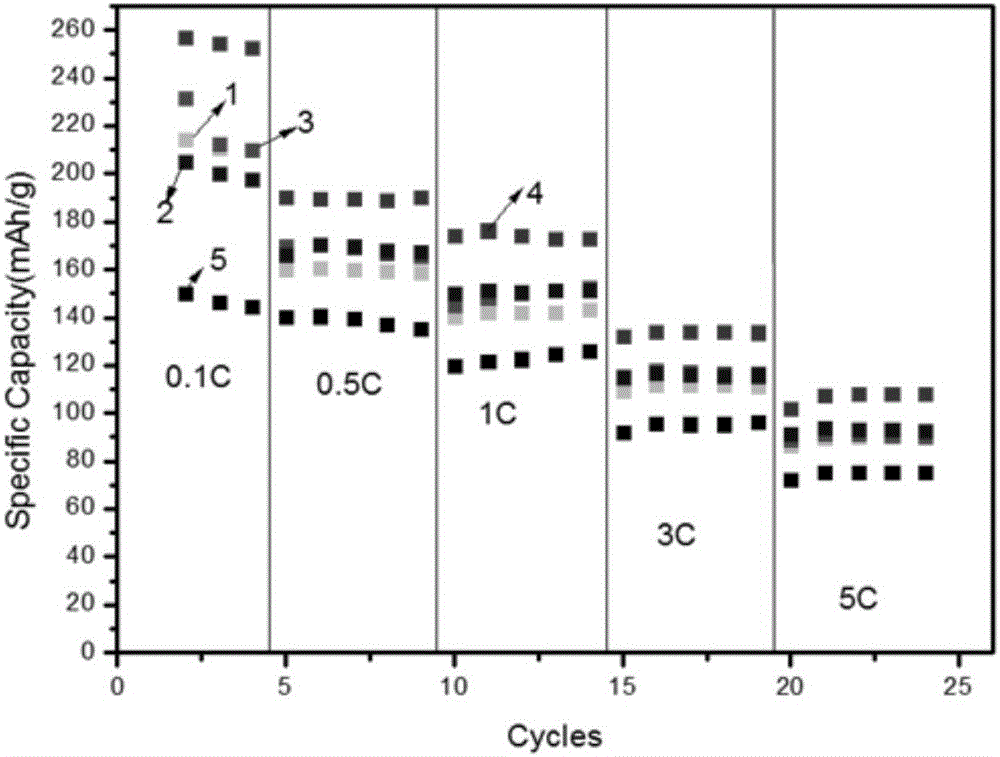
[0029] 1) Weigh 296g of lithium carbonate, 400g of titanium dioxide and 5.6g of zirconium dioxide, add 14g of polyvinyl alcohol, add water to form a dispersion with a solid content of 20%, and ball mill for 4 hours;
[0030] 2) Spray drying to remove moisture, and the obtained solid was calcined at 700° C. for 6 hours in an air atmosphere to obtain a primary lithium titanate material;
[0031] 3) Weigh 2.6g of graphene-coated material, 2.5g of phosphoric acid and 2.7g of alumina and disperse them into water, add the primary lithium titanate material obtained in step 2), add water to adjust the solid content to 25%, and ball mill for 3 hours;
[0032] 4) Spray drying to remove moisture, and the obtained solid was calcined at 700° C. for 3 hours under a nitrogen atmosphere to obtain a graphene-coated lithium titanate material with a carbon content of 0.45%.
[0033] from figure 1 From the scanned picture shown, it can be seen that the spherical shape is better, which is conduci...
Embodiment 2
[0035] 1) Weigh 240g of lithium hydroxide, 1200g of titanium dioxide and 14.4g of zirconium dioxide, add 50g of polyvinyl alcohol, add water to form a dispersion with a solid content of 25%, and ball mill for 6 hours;
[0036] 2) Spray drying to remove moisture, and the obtained solid was calcined at 800° C. for 7 hours in an air atmosphere to obtain a primary lithium titanate material;
[0037] 3) Weigh 13g of graphene oxide coating material, 5.4g of lithium fluoride and 20.4g of phosphoric acid and disperse into water, add the primary lithium titanate material obtained in step 2), add water to adjust the solid content to 30%, and ball mill for 3.5 hours ;
[0038] 4) Spray drying to remove moisture, and the obtained solid was calcined at 850° C. for 3.5 hours under a nitrogen-hydrogen mixed gas atmosphere to obtain a graphene-coated lithium titanate material with a carbon content of 0.85%.
Embodiment 3
[0040] 1) Weigh 592g of lithium carbonate, 720g of titanium dioxide and 26.2g of lithium fluoride, add 28g of polyvinyl alcohol, add water to form a dispersion with a solid content of 25%, and ball mill for 5 hours;
[0041] 2) Spray drying to remove moisture, and the obtained solid was calcined at 850° C. for 7 hours in an air atmosphere to obtain a primary lithium titanate material;
[0042] 3) Weigh 28g of multilayer graphene-coated material, 12.1g of phosphoric acid and 17.8g of molybdenum trioxide and disperse them in water, add the primary lithium titanate material obtained in step 2), and add water to adjust the solid content to 30%, and ball mill for 5 Hour;
[0043] 4) Spray drying to remove moisture, and the obtained solid was calcined at 800° C. for 3 hours under a nitrogen atmosphere to obtain a graphene-coated lithium titanate material with a carbon content of 2.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com