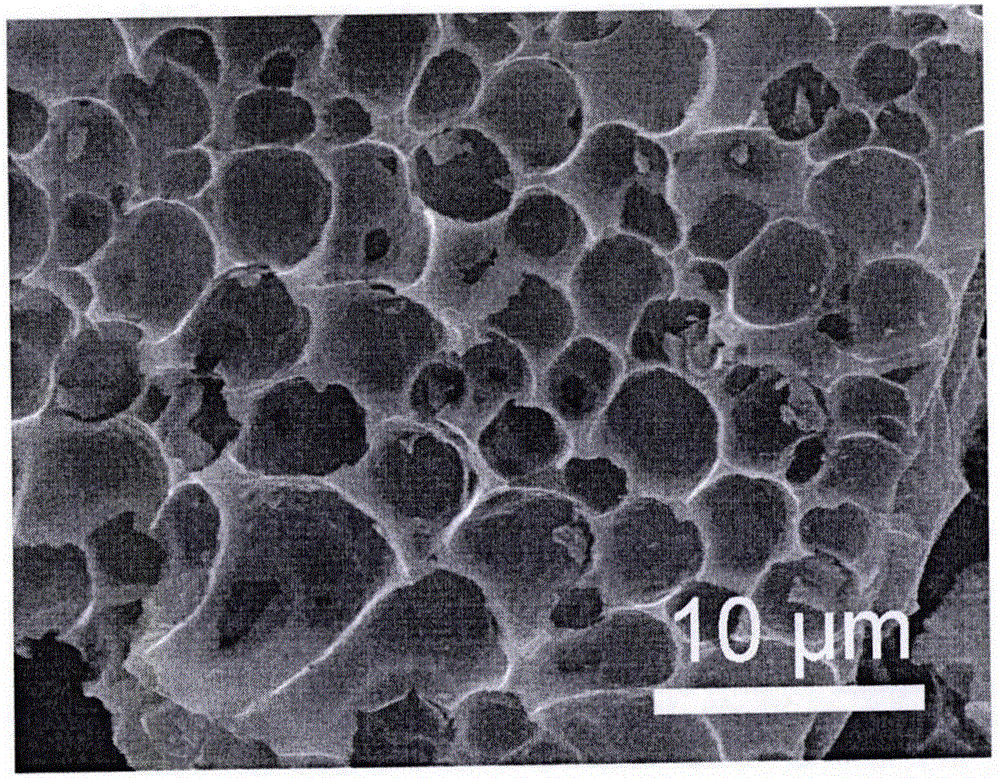
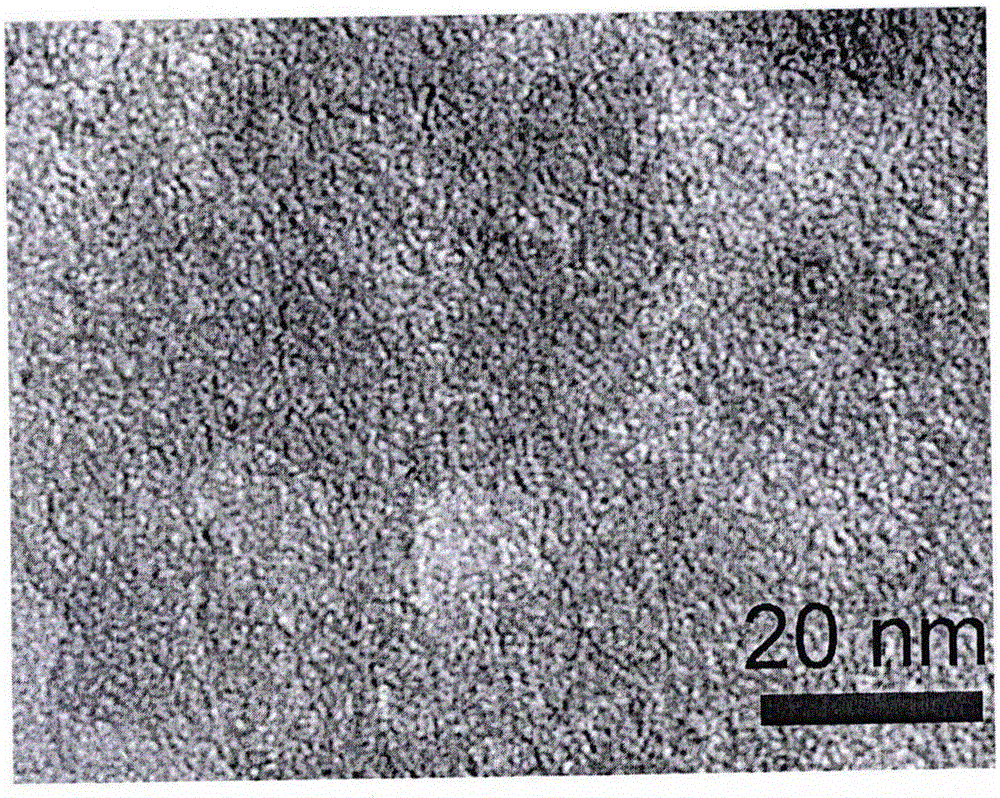
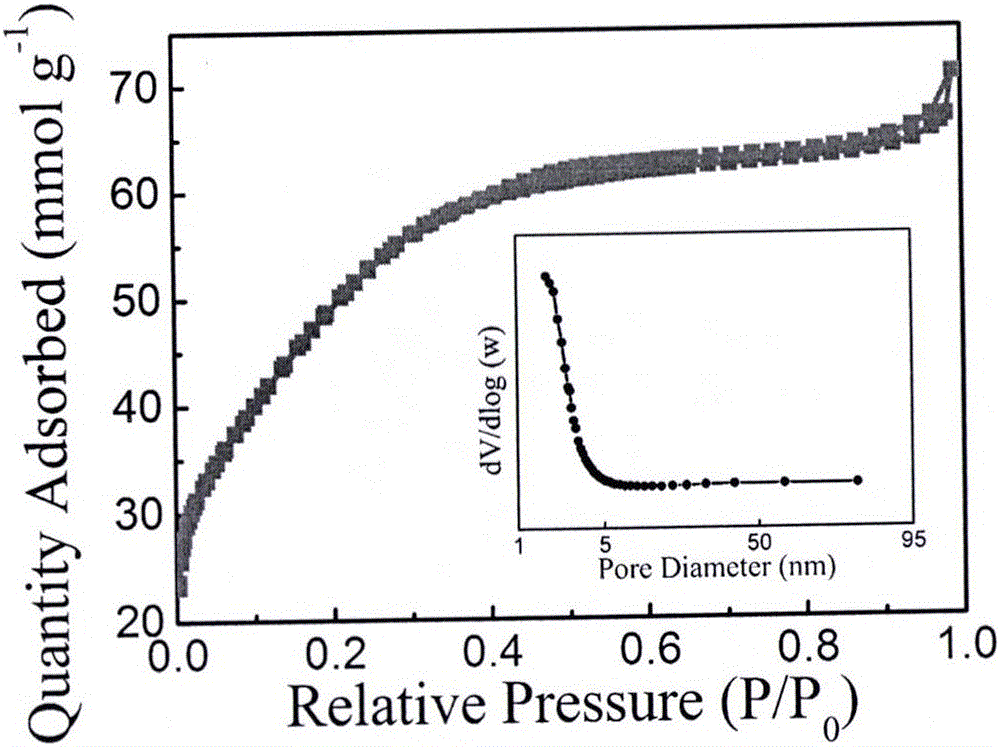
Preparation method of porous carbon aerogel with super-high specific surface area
A technology of ultra-high specific surface area and carbon airgel, which is applied in the direction of electrical components, battery electrodes, non-aqueous electrolyte batteries, etc., to achieve good cycle stability, simple preparation process, and high specific capacity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1) Drop the iota-type carrageenan aqueous solution with a mass fraction of 2% into the ethanol solution of ferric chloride with a concentration of 1M, wash with deionized water, and prepare a carrageenan-iron hydrogel.
[0024] 2) preparing the carrageenan-iron hydrogel into an aerogel by freeze-drying;
[0025] 3) Carrageenan-iron aerogel is calcined at 400°C for 1 hour in a tube furnace under an argon atmosphere to obtain a carbon aerogel-iron sulfide nanocomposite;
[0026] 4) remove the iron sulfide in the carbon aerogel-iron sulfide nanocomposite material with an aqueous hydrochloric acid solution with a concentration of 2M to obtain the carbon aerogel;
[0027] 5) The carbon aerogel is activated with potassium hydroxide, and the mass ratio of potassium hydroxide to the carbon aerogel is 4:1. The activation process is calcination at 800° C. for 1 hour in a nitrogen atmosphere. Obtain super high specific surface porous carbon deposition airgel;
[0028] 6) The el...
Embodiment 2
[0030] 1) Drop the iota-type carrageenan aqueous solution with a mass fraction of 2% into the ethanol solution of ferric chloride with a concentration of 1M, wash with deionized water, and prepare a carrageenan-iron hydrogel.
[0031] 2) preparing the carrageenan-iron hydrogel into an aerogel by freeze-drying;
[0032] 3) Carrageenan-iron aerogel was calcined at 300°C for 1 hour in a tube furnace under an argon atmosphere to obtain a carbon aerogel-iron sulfide nanocomposite;
[0033] 4) remove the iron sulfide in the carbon aerogel-iron sulfide nanocomposite material with an aqueous hydrochloric acid solution with a concentration of 2M to obtain the carbon aerogel;
[0034] 5) The carbon aerogel is activated with potassium hydroxide, and the mass ratio of potassium hydroxide to the carbon aerogel is 4:1. The activation process is calcination at 800° C. for 1 hour in a nitrogen atmosphere. Obtain super high specific surface porous carbon deposition airgel;
[0035] 6) The e...
Embodiment 3
[0037] 1) Drop the iota-type carrageenan aqueous solution with a mass fraction of 2% into the ethanol solution of ferric chloride with a concentration of 1M, wash with deionized water, and prepare a carrageenan-iron hydrogel.
[0038] 2) preparing the carrageenan-iron hydrogel into an aerogel by freeze-drying;
[0039] 3) Carrageenan-iron aerogel is calcined at 500°C for 1 hour in a tube furnace under an argon atmosphere to obtain a carbon aerogel-iron sulfide nanocomposite;
[0040] 4) remove the iron sulfide in the carbon aerogel-iron sulfide nanocomposite material with an aqueous hydrochloric acid solution with a concentration of 2M to obtain the carbon aerogel;
[0041] 5) The carbon aerogel is activated with potassium hydroxide, and the mass ratio of potassium hydroxide to the carbon aerogel is 4:1. The activation process is calcination at 800° C. for 1 hour in a nitrogen atmosphere. Obtain super high specific surface porous carbon deposition airgel;
[0042] 6) The el...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com