Recombinant bacillus subtilis for producing pullulanase and construction thereof
A technology of Bacillus subtilis and pullulanase, which is applied in the field of microbial fermentation to produce pullulanase, can solve problems such as product safety threats, and achieve the effect of improving the ability to produce extracellular pullulanase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the acquisition of pullulanase gene pul
[0027] Bacillus naganoensis CCTCC M 2012388 Medium: CaCl 2 0.25g / L, MgSO 4 ·7H 2 O 0.5g / L, (NH 4 ) 2 SO 4 0.2g / L, yeast extract 2g / L, anhydrous glucose 5g / L, KH 2 PO 4 3g / L, 1mL / L inorganic salt solution, prepared with double distilled water, pH 5.0. Inorganic salt solution: ZnSO 4 ·7H 2 O 0.1g / L, MnCl 2 ·H 2 O0.03g / L, H 3 BO 3 0.3g / L, CoCl 2 ·6H 2 O 0.2g / L, CuCl 2 2H 2 O 0.01g / L, NiCl 2 ·6H 2 O 0.02g / L, Na 2 MoO 4 2H 2 O 0.03g / L.
[0028] The Bacillus nagano strain was inoculated into a 250 mL shake flask containing 25 mL of culture medium, and cultured with shaking at 37° C. and 200 rpm for 72 h. After the cultivation, the cells were centrifuged and washed twice with physiological saline, and the cells were collected to extract genomic DNA using a genomic DNA extraction kit Genomic DNA Extraction Miniprep System (VIOGENE Company).
[0029] Synthetic Primer 1: 5'-CG GAATTC GATGGGAACACCA...
Embodiment 2
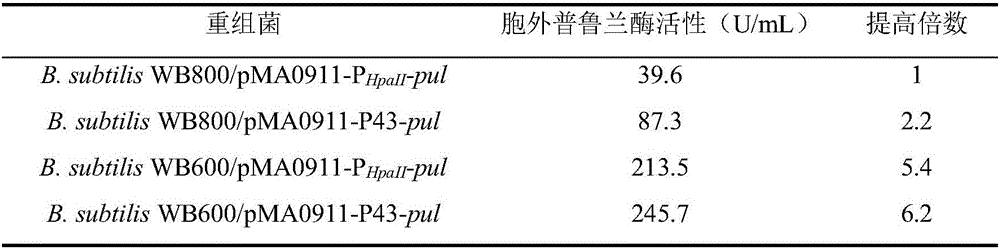
[0035] Example 2: Recombinant Bacillus subtilis B. subtilis WB800 / pMA0911-P HpaII -build of pul
[0036] (1) Double digestion of target gene pul and plasmid pMA0911
[0037] Plasmid pMA0911 was extracted using the plasmid extraction kit Plasmid Mini Kit (OMEGA BIO-TEK).
[0038] Add water, buffer, PCR product or plasmid DNA, and restriction endonuclease into the Eppendorf tube in the order, cover the tube cap, mix gently, and centrifuge in a centrifuge for 30 seconds to concentrate the liquid at the bottom of the tube. Water bath at 37°C for 3h, add 1 / 10 Loading Buffer to the tube or place the tube at 65°C for 10min to terminate the enzyme digestion reaction. The digested product was analyzed by agarose gel electrophoresis, and the target fragment was recovered by using Gel extraction Kit (OMEGA BIO-TEK Company), and concentrated appropriately.
[0039] Enzyme digestion reaction system composition: 10×K Buffer 10 μL, genomic DNA 80 μL, restriction enzyme EcoRI 2.5 μL, restr...
Embodiment 3
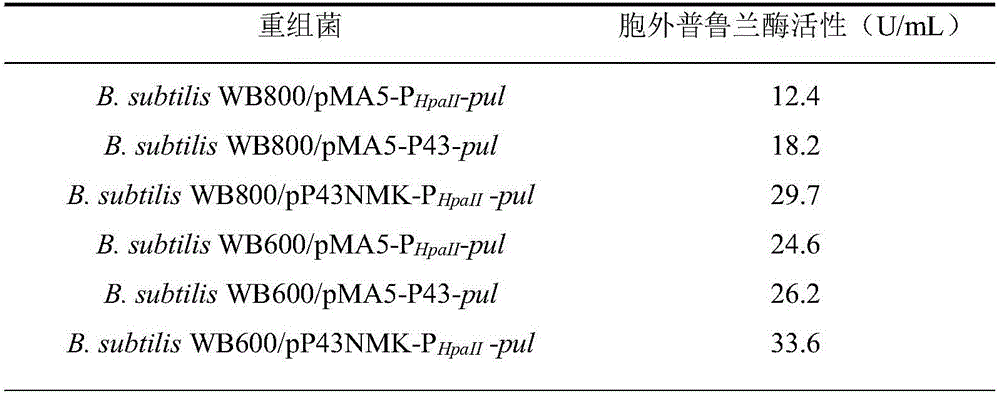
[0047] Example 3: Promoter optimization
[0048] (1) Gene synthesis of P43 promoter
[0049] The P43 promoter sequence (GenBank: EF473728.1) 426bp was searched in NCBI, and single enzyme cutting sites BstxI and EcoRI were introduced on both sides of the synthetic gene, and the sequence to be synthesized was sent to Shanghai Sangon Bioengineering Co., Ltd. Gene synthesis was carried out, and the synthesized gene fragment was cloned in the strain E.coil DH5α / pUC57-P43.
[0050] Utilize the plasmid extraction kit Plasmid Mini Kit (OMEGA BIO-TEK company) to extract from bacterial strain E.coilDH5α / pUC57-P43 and E.coli JM109 / pMA0911-P respectively HpaII Extract plasmids pUC57-P43 and pMA0911-P from -pul HpaII -pul.
[0051] (2) Plasmid pUC57-P43 and plasmid pMA0911-P HpaII Double digestion of -pul
[0052] According to water, buffer, plasmid pUC57-P43 or plasmid pMA0911-P HpaII Add -pul, restriction endonuclease EcoRI and BstXI (NEB company) to the Eppendorf tube in sequence,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com