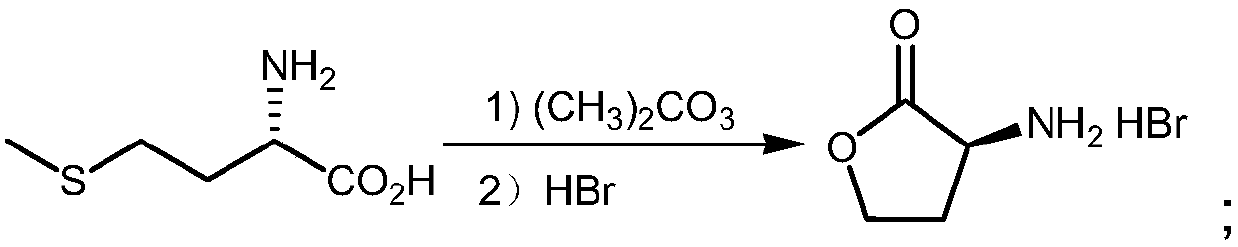A kind of preparation method of selenomethionine
A technology of selenomethionine and methionine, which is applied in the field of preparation of selenomethionine, can solve the problems of high equipment requirements and difficult operation, and achieve the effects of simple equipment, increased yield, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
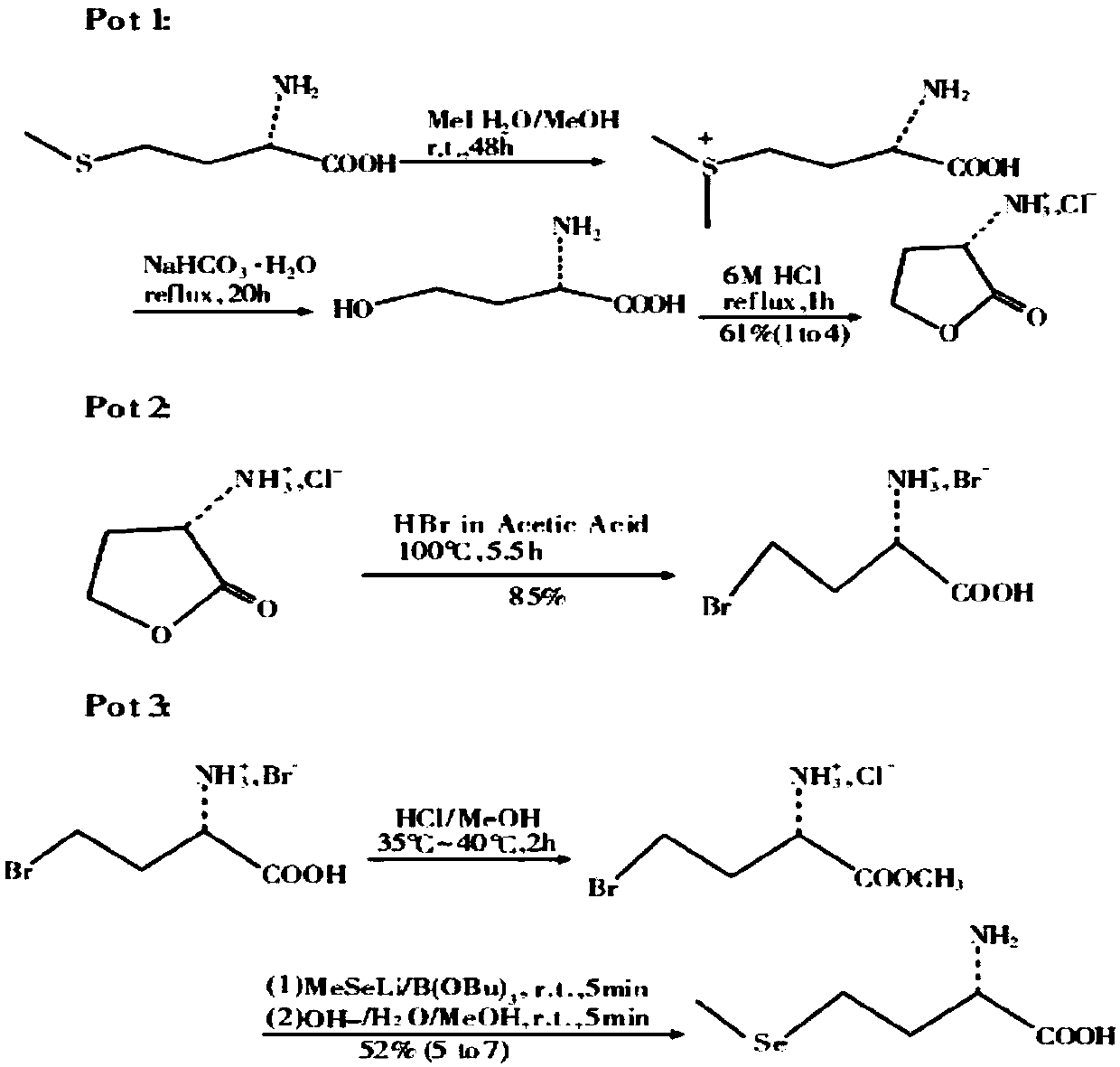
[0035] (a) Synthesis of L-α-amino-γ-butyrolactone hydrobromide: in a 50L reactor, add 3.4kgL-methionine, 2.8kg dimethyl carbonate, 12.5kg ethanol (density is 0.998g / cm 3 ) and 12.5kg of water, add 6.12kg of glacial acetic acid (with a density of 1.05g / cm 3 ) and 3.4kg of bromoacetic acid, then heated to 70°C for reflux for 5 hours, distilled under reduced pressure, and then added 6.8kg (density of 1.38g / cm 3 ) 40% hydrobromic acid, heated to 50°C for 2h, cooled to 20°C, cooled to crystallize, filtered to obtain white crystalline solid L-α-amino-γ-butyrolactone hydrobromide (intermediate 1), The yield is 95%;
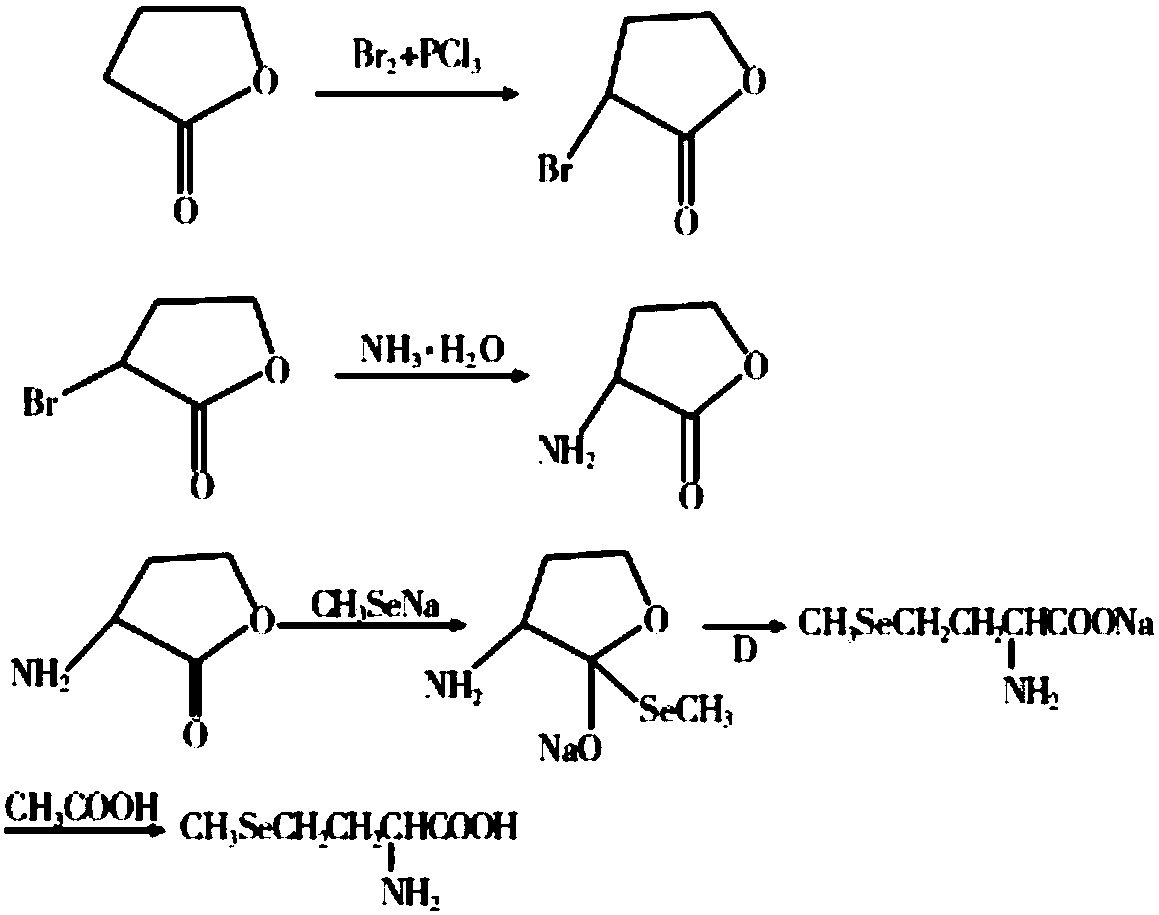
[0036] (b) Synthesis of sodium methyl selenide: In a 50L reaction kettle, add 20kg of 20% sodium hydroxide solution, then add 4.7kg of selenium powder, 3.8kg of hydrazine hydrate, and stir for 48 hours at 20°C. Then add 5.7kg dimethyl carbonate, stir and react at 20°C for 12h, after the reaction is over, let it stand for stratification, separate the lower organic phase...
Embodiment 2
[0039] (a) Synthesis of L-α-amino-γ-butyrolactone hydrobromide: in a 50L reactor, add 5kgL-methionine, 4kg dimethyl sulfate, 10kg isopropanol (density is 0.998g / cm 3 ) and 10kg of water, add 6.5kg of glacial acetic acid (with a density of 1.05g / cm 3 ) and 3.5kg of bromoacetic acid, then heated to 90°C for reflux reaction for 1h, distilled under reduced pressure, and then added 2kg (with a density of 1.38g / cm 3 ) 40% hydrobromic acid, heated to 70°C for 0.5h, cooled to 25°C, cooled to crystallize, filtered to obtain white crystalline solid L-α-amino-γ-butyrolactone hydrobromide (intermediate 1) , the yield is 92%;
[0040] (b) Synthesis of sodium methyl selenide: In a 50L reaction kettle, add 12kg of 20% sodium hydroxide solution, then add 2.4kg of selenium powder, 2kg of hydrazine hydrate, and stir for 24 hours at 25°C. Then add 3kg of dimethyl sulfate, stir and react at 20°C for 4h, after the reaction is over, let it stand for stratification, separate the lower organic phas...
Embodiment 3
[0043] (a) Synthesis of L-α-amino-γ-butyrolactone hydrobromide: in a 100L reactor, add 15kgL-methionine, 12kg dimethyl carbonate, 15kg isopropanol (density is 0.998g / cm 3 ) and 15kg of water, add 28.8kg of glacial acetic acid (with a density of 1.05g / cm 3 ) and 16kg of bromoacetic acid, then heated to 80°C for reflux for 3h, distilled under reduced pressure, and then added 30kg (with a density of 1.38g / cm 3 ) 40% hydrobromic acid, heated to 60°C for 1.5h, cooled to 25°C, cooled to crystallize, filtered to obtain white crystalline solid L-α-amino-γ-butyrolactone hydrobromide (intermediate 1) , the yield is 90%;
[0044] (b) Synthesis of sodium methyl selenide: In a 100L reactor, add 20kg of 20% sodium hydroxide solution, then add 4.8kg of selenium powder, 4kg of hydrazine hydrate, and stir for 26 hours at 23°C. Then add 6kg dimethyl sulfate, stir and react at 23°C for 10h, after the reaction is over, let it stand for stratification, separate the lower organic phase, then add ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com