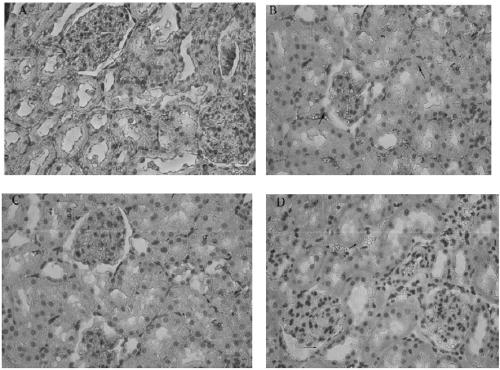
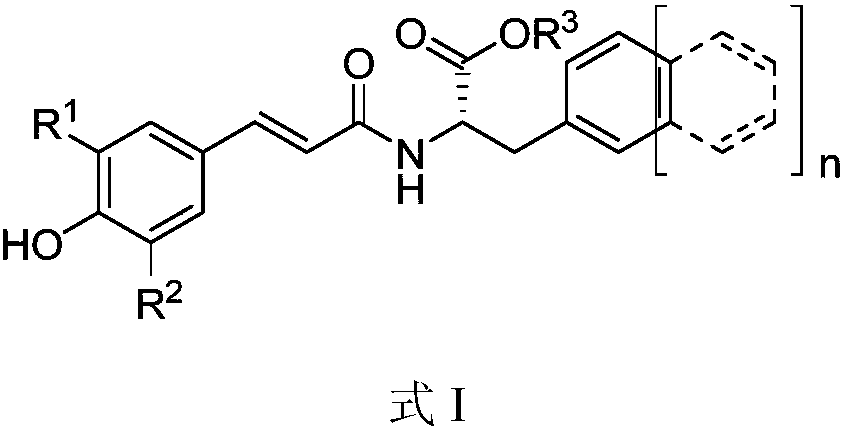
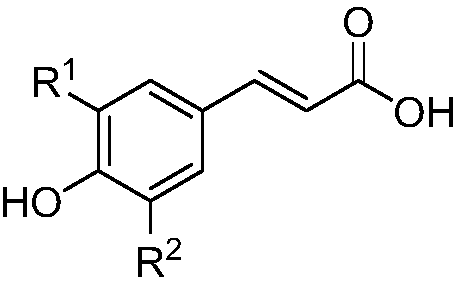
A kind of medicine for preventing and treating diabetes and diabetic nephropathy and its synthesis method and application
A technology of diabetic nephropathy and synthetic method, which is applied in the field of related drugs, can solve problems such as unsatisfactory activity and structure-activity relationship research, and achieve the effect of optimizing the synthetic route, easily obtaining synthetic raw materials, and easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0047] The synthesis of example 1 caffeic acid-phenylalanine amide compound (CP1 for short, as shown in formula II)
[0048]
[0049] 1) Weigh 90mg (0.5mmol) of caffeic acid and add it to a 10mL round-bottomed flask, add 5mL of trichloroethane dropwise to the round-bottomed flask to dissolve the caffeic acid;
[0050] 2) Add 208 μL (about 1.5 mmol) of triethylamine to the round bottom flask, and stir for 10 min in an ice-water bath to bring the temperature of the system to 0°C;
[0051] 3) Add 143 mg (0.75 mmol) of EDCI (1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride) and DMAP ( 4-Dimethylaminopyridine) 91.5mg (0.75mmol), after 20min, add L-phenylalanine hydrochloride 101mg (0.5mmol) to the round-bottomed flask, the temperature rose to 40°C, and continued stirring Respond for 24 hours;
[0052] 4) After the reaction was completed, the solvent was vacuum-dried (rotary evaporation, -0.08MPa, 45°C), and 20 mL of ethyl acetate was added to shake well, and filtered...
example 2
[0053] The synthesis of example 2 ferulic acid-phenylalanine isopropyl ester (CP2 for short, as shown in formula III)
[0054]
[0055] 1) Weigh 97 mg (0.5 mmol) of ferulic acid and add it to a 10 mL round-bottomed flask, add 6 mL of DCM (dichloromethane) dropwise to the round-bottomed flask to dissolve the ferulic acid;
[0056] 2) Add 205 μL (about 1.5 mmol) of diisopropylamine to the round bottom flask, and stir for 15 minutes in an ice-water bath to make the temperature of the system reach 0°C;
[0057] 3) Add NMM (N-methylmorpholine) 101mg (1mmol) and IBCF (isobutyl chloroformate) 135mg (1mmol) to the round-bottomed flask under ice-water bath and stirring conditions, and pour into the round-bottomed flask after 40min Then add 101 mg (1 mmol) of L-phenylalanine isopropyl ester hydrochloride, raise the temperature to 80°C, and continue to react under stirring conditions for 18 hours;
[0058] 4) After the reaction was completed, the solvent was vacuum-dried (rotary evap...
example 3
[0059] Example 3 Synthesis of 3-acetyl-caffeic acid-β-naphthylalanine benzyl ester (CP3 for short, shown in formula IV)
[0060]
[0061] 1) Weigh 111mg (0.5mmol) of the raw material 3-acetyl-caffeic acid (structural formula is as follows) into a 10mL round-bottomed flask, and add 5mL of toluene dropwise into the round-bottomed flask to dissolve the raw material;
[0062]
[0063] 2) Add 70 μL (about 0.5 mmol) of triethylamine to the round-bottomed flask, and stir for 20 minutes in an ice-water bath to make the temperature of the system reach 0°C;
[0064] 3) Add EDCI 95mg (0.5mmol) and HOBt67.3mg (0.5mmol) to the round bottom flask under ice-water bath and stirring conditions, and then add β-L-naphthylalanine benzyl ester to the round bottom flask after 30min 183mg (0.6mmol), the temperature was raised to 60°C, and the reaction was continued for 48 hours under stirring;
[0065] 4) After the reaction was completed, the solvent was vacuum-dried (rotary evaporation, -0.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com