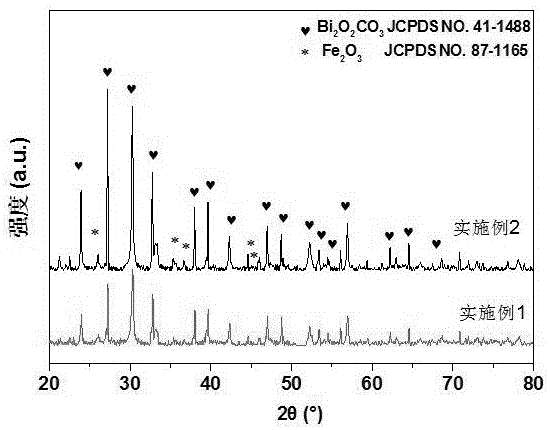
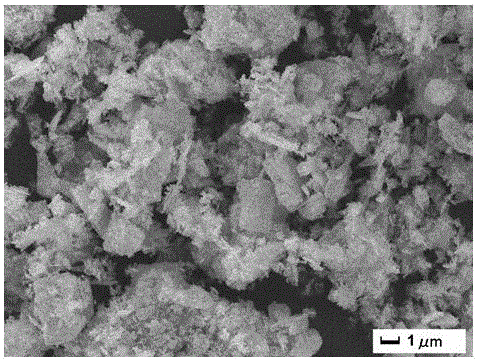
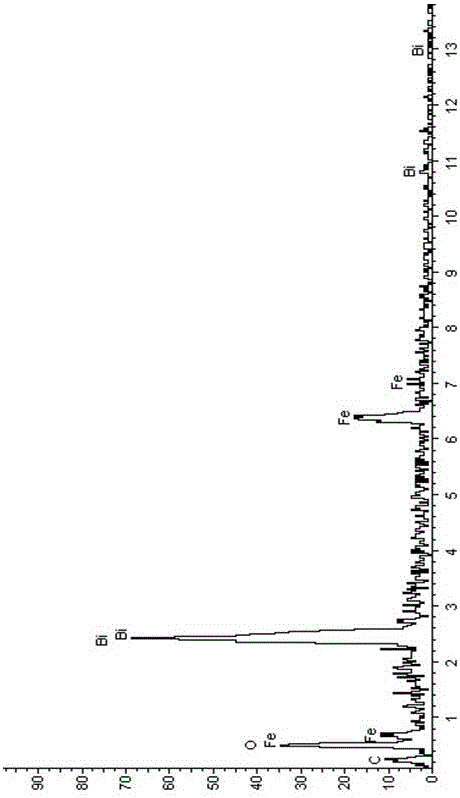
Preparation method of iron sesquioxide-bismuthylcarbonate composite light catalyst
A technology of ferric oxide and bismuth oxycarbonate, which is used in physical/chemical process catalysts, chemical instruments and methods, water treatment of special compounds, etc., can solve the problems of high price of composite components, complex synthesis steps, and unfavorable wide application. , to achieve the effect of remarkable photocatalytic effect, low operating cost and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Weigh 0.433g FeCl 3 ·6H 2 O was dissolved in 2.4 g absolute ethanol and stirred until FeCl 3 ·6H 2 O was completely dissolved, and 0.537 g of deionized water was added to the solution, followed by 0.649 g of propylene oxide, and allowed to stand for 12 h to form a brown-red iron-based gel.
[0030] The gel was placed in a 25 mL polytetrafluoroethylene autoclave, and 0.8 g Bi(NO 3 ) 2 .5H 2 O and 10mL dilute nitric acid (4 mol·L -1 ) was used as the reaction solvent, and the hydrothermal reaction was carried out for 12 h at the temperature of the mixed system at 180 °C.
[0031] After the reaction, it was naturally cooled to room temperature, and the reacted sample was taken out and washed three times with ethanol at 50°C. The washed samples were placed in a blast drying oven at a controlled temperature of 60°C and dried for 6 hours to obtain ferric oxide-bismuth oxycarbonate.
Embodiment 2
[0033] Weigh 0.866g FeCl 3 ·6H 2 O was dissolved in 6.4 g of absolute ethanol and stirred until FeCl 3 ·6H 2 O was completely dissolved, and 2.0 g of deionized water was added to the solution, followed by 1.3 g of propylene oxide, and allowed to stand for 20 h to form a brown-red iron-based gel.
[0034] The gel was placed in a 50 mL polytetrafluoroethylene autoclave, and 1.6 g of Bi(NO 3 ) 2 .5H 2 O and 20mL dilute nitric acid (4 mol·L -1 ) was used as the reaction solvent, and the hydrothermal reaction was carried out for 24 hours under the condition that the temperature of the mixed system was 180°C.
[0035] After the reaction, it was naturally cooled to room temperature, and the reacted sample was taken out and washed three times with ethanol at 50°C. The washed sample was placed in a forced air drying oven at a controlled temperature of 80°C and dried for 8 hours to obtain ferric oxide-bismuth oxycarbonate.
Embodiment 3
[0037] Weigh 0.433g FeCl 3 ·6H 2 O was dissolved in 4.0 g absolute ethanol and stirred until FeCl 3 ·6H 2 O was completely dissolved, and 0.423g of deionized water was added to the solution, and then 0.65g of propylene oxide was added and allowed to stand for 14h to form a brown-red iron-based gel. The gel was placed in a 25 mL polytetrafluoroethylene autoclave, and 1.2 g of Bi(NO 3 ) 2 .5H 2 O and 14mL dilute nitric acid (4 mol·L -1 ) was used as the reaction solvent, and the hydrothermal reaction was carried out for 24 hours under the condition that the temperature of the mixed system was 180°C.
[0038] After the reaction, it was naturally cooled to room temperature, and the reacted sample was taken out and washed three times with ethanol at 50°C. The washed samples were placed in a blast drying oven at a controlled temperature of 80°C and dried for 10 hours to obtain ferric oxide-bismuth oxycarbonate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com