Preparing method of elastic degradable biomedical material
A technology for biomedical materials and degradable polymers, applied in the field of biomedical material preparation, can solve the problems of large elastic deformation, poor controllability of degradation rate, limited application, poor strength and extensibility, affecting wound healing and tissue repair, etc. The effect of controllable degradation rate, good breaking strength and elasticity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
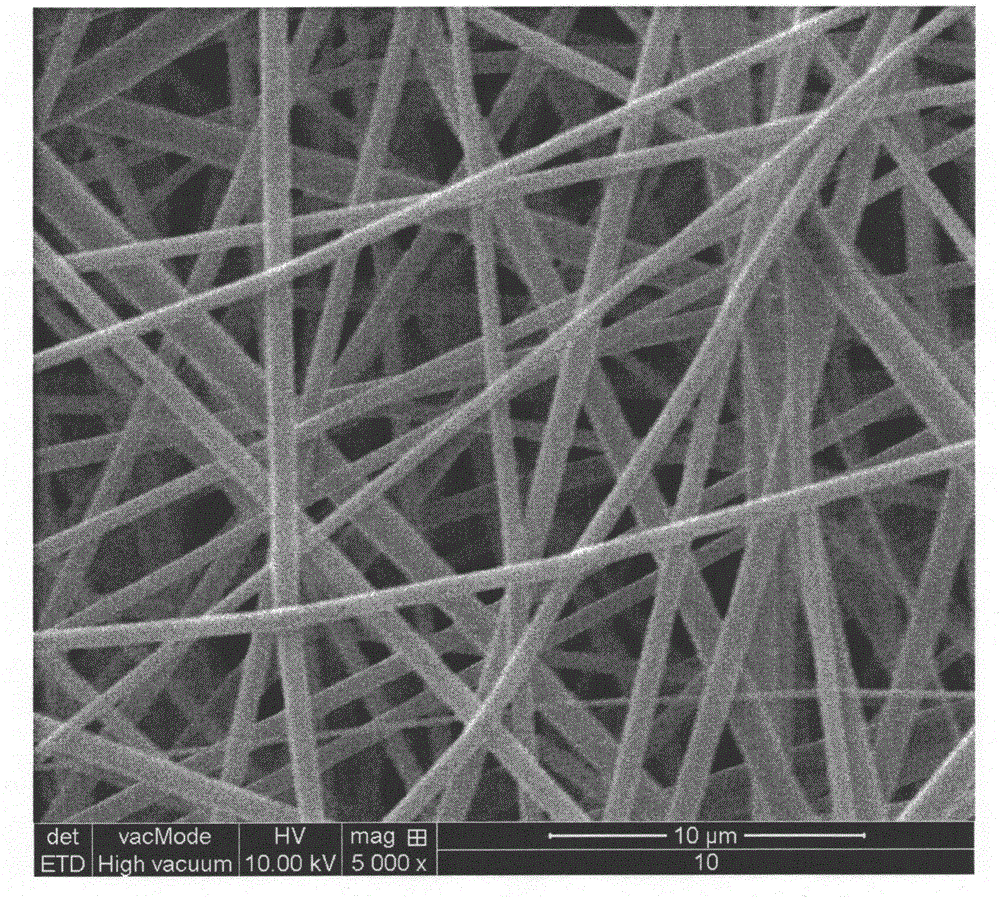
Image
Examples
Embodiment 1
[0028] A method for preparing an elastic degradable biomedical material, the specific steps are:
[0029] (1) Synthesis of POC (poly-1,8-octanediol-citrate) elastomer by melt condensation polymerization of 1,8-octanediol and citric acid: 1,8-octanediol and citric acid molar Weigh it at a ratio of 1:1, put it into a three-necked round bottom flask, and stir it in an oil bath under normal pressure and a temperature of 160-165°C for 15 minutes to make the reaction monomer 1,8-octanediol and citric acid Melt completely, and then drop the temperature to 140-145°C for 40-60 minutes to melt and polycondense until the magnetic stirring is difficult (close to the gel point) to obtain POC, and then dissolve the POC in 50ml of absolute ethanol and deionize Precipitate in water (remove unreacted monomer by dissolving), then collect POC and vacuum freeze-dry for 24 hours to obtain purified POC.
[0030] (2) Weigh 0.36g POC and 0.54g PLA (POC / PLA: 40 / 60) with ME104E Mettler-Torley electron...
Embodiment 2
[0035] A method for preparing an elastic degradable biomedical material, the specific steps are:
[0036] (1) is the same as step (1) in Example 1.
[0037] (2) Weigh 0.225g POC and 0.675g PLA (POC / PLA: 25 / 75) with ME104E Mettler-Torley electronic balance, mix and dissolve in 10ml of hexafluoroisopropanol solvent, make it fully stirred until Dissolve completely, obtain the POC / PLA mixed spinning solution that the total concentration of POC and PLA is 9% (g / ml);
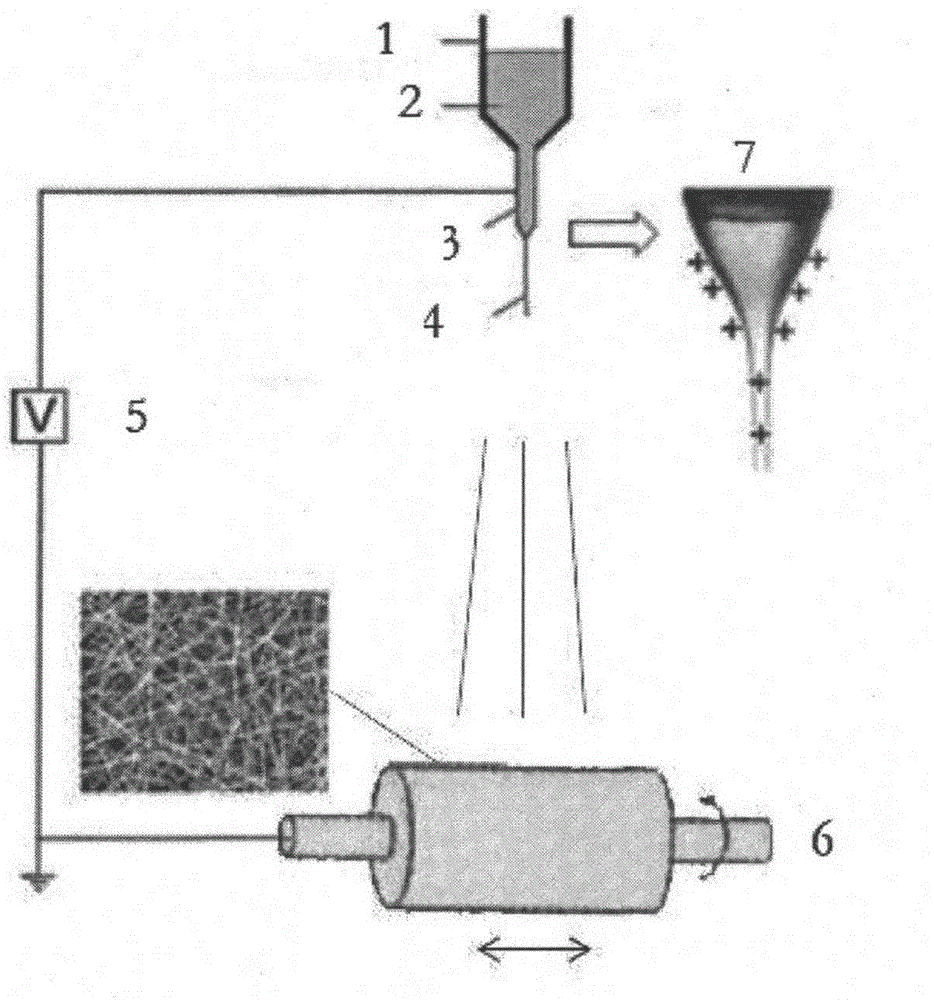
[0038] (3) Use electrospinning equipment for electrospinning, such as figure 1As shown, the electrospinning equipment includes a syringe 1 (plastic syringe) with a capacity of 10ml, a high-voltage power supply 5 and a receiving device 6. The bottom end of the syringe 1 is provided with a 21G flat needle 3, and the flat needle 3 is located on the receiving device. Above the device 6, the positive pole of the high voltage power supply 5 is connected to the flat needle 3, and the negative pole is grounded. The voltage ...
Embodiment 3
[0042] A method for preparing an elastic degradable biomedical material, the specific steps are:
[0043] (1) is the same as step (1) in Example 1.
[0044] (2) Weigh 0.09g POC and 0.81g PLA (POC / PLA: 10 / 90) with ME104E Mettler-Torley electronic balance, mix and dissolve them in 10ml of hexafluoroisopropanol solvent, and stir them thoroughly until Dissolve completely, obtain the POC / PLA mixed spinning solution that the total concentration of POC and PLA is 9% (g / ml);
[0045] (3) Use electrospinning equipment for electrospinning, such as figure 1 As shown, the electrospinning equipment includes a syringe 1 (plastic syringe) with a capacity of 10ml, a high-voltage power supply 5 and a receiving device 6. The bottom end of the syringe 1 is provided with a 21G flat needle 3, and the flat needle 3 is located on the receiving device. Above the device 6, the positive pole of the high voltage power supply 5 is connected to the flat needle 3, and the negative pole is grounded. The v...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com