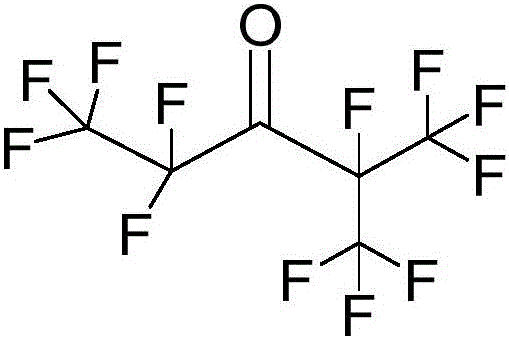
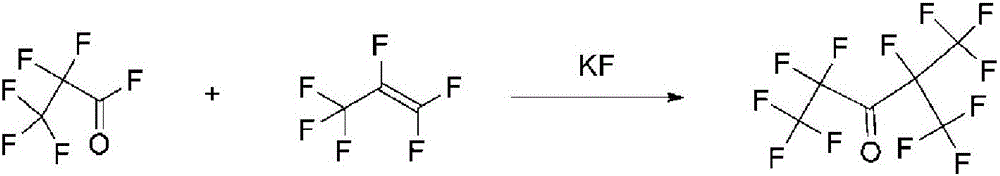
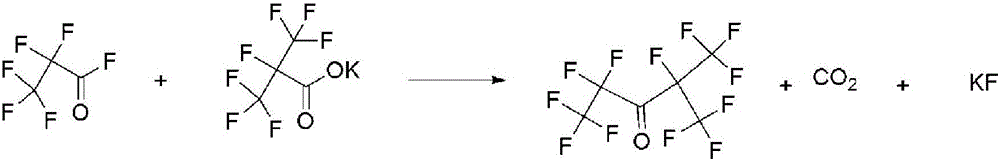
Method of preparing pentafluoroethyl isopropyl ketone
A technology of perfluoroethyl isopropyl ketone and hexafluoropropylene, which is applied in the field of preparation of perfluoroethyl isopropyl ketone, can solve the problems of low selectivity, difficult manipulation, and many by-products, and achieve a high degree of automation , easy operation and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] A method for preparing perfluoroethyl isopropyl ketone, comprising the steps:
[0061] (1) Mix 540L 1,1,2-trichlorotrifluoroethane (Freon 113), 300L sodium hypochlorite aqueous solution (active chlorine content 10%), 2Kg tetrabutylammonium chloride and 2Kg perfluoroalkyl polyether (FN -6810) into a 1000L autoclave with stirring (type 316 stainless steel). The reaction system was cooled to minus 10°C, the air in the reaction kettle was evacuated, 260Kg of hexafluoropropylene (measured by weight loss method) was introduced, the temperature was controlled at -10°C to -5°C, and the reaction was performed for 2 hours. After the reaction was stopped, let stand for 15 minutes. , Sampling detection, gas chromatography results show that the conversion rate of hexafluoropropylene is 97%, and the yield of hexafluoropropylene oxide is 93%. The organic phase (1,1,2-trichlorotrifluoroethane) was transferred to another reaction kettle from the lower discharge port of the reaction ket...
Embodiment 2
[0064] A method for preparing perfluoroethyl isopropyl ketone, the preparation method is the same as that in Example 1, the difference is only that: the phase transfer catalyst tetrabutylammonium chloride in step (1) is replaced with trioctyl methyl chloride ammonium chloride, and the solvent in step (2), dimethyl ether oxalate, was replaced with acetonitrile.
[0065] After testing, after the reaction in step (1), the conversion rate of hexafluoropropylene was 99%, and the yield of hexafluoropropylene oxide was 94%; after the reaction in step (2), the conversion rate of hexafluoropropylene was 100%, The yield of perfluorohexanone is 72%, the main impurity is hexafluoropropylene dimer
[0066] (22.3%).
Embodiment 3
[0068] This example is the same as Example 1, the difference is only that the solvent in step (2) is replaced with N,N-dimethylformamide. After the reaction in step (2), the conversion rate of hexafluoropropylene was 100%, the yield of perfluorohexanone was 76%, and the main impurity was hexafluoropropylene dimer (18.3%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com