Preparation method of fluoromethyl ketone peptide series compounds
A technology of compound and carboxyl group, which is applied in the field of compound preparation, can solve the problems of unfavorable industrial production, strong corrosion of reaction reagents, and limited industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
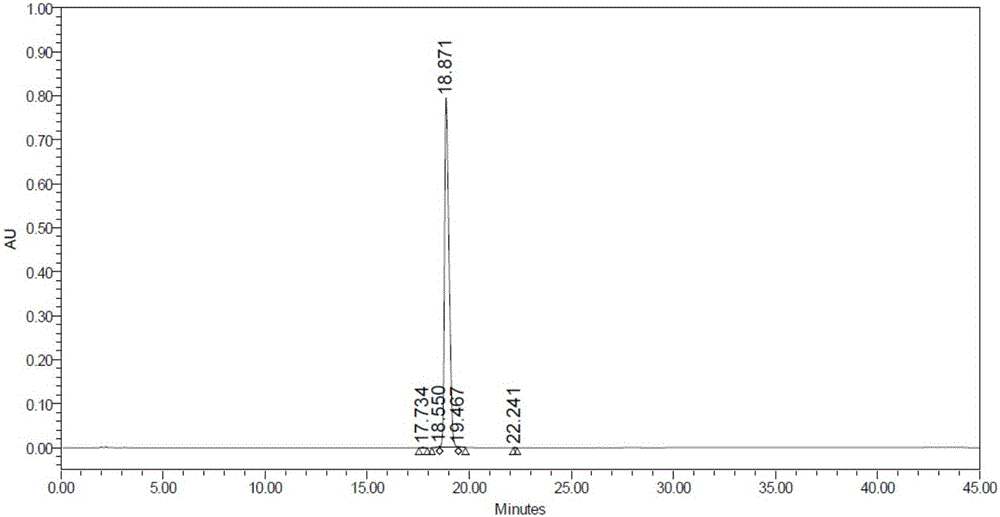
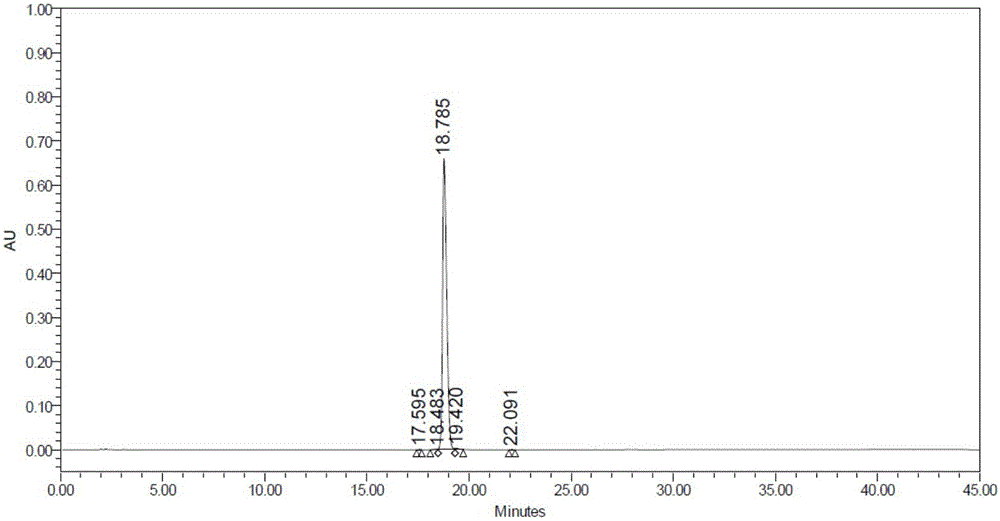
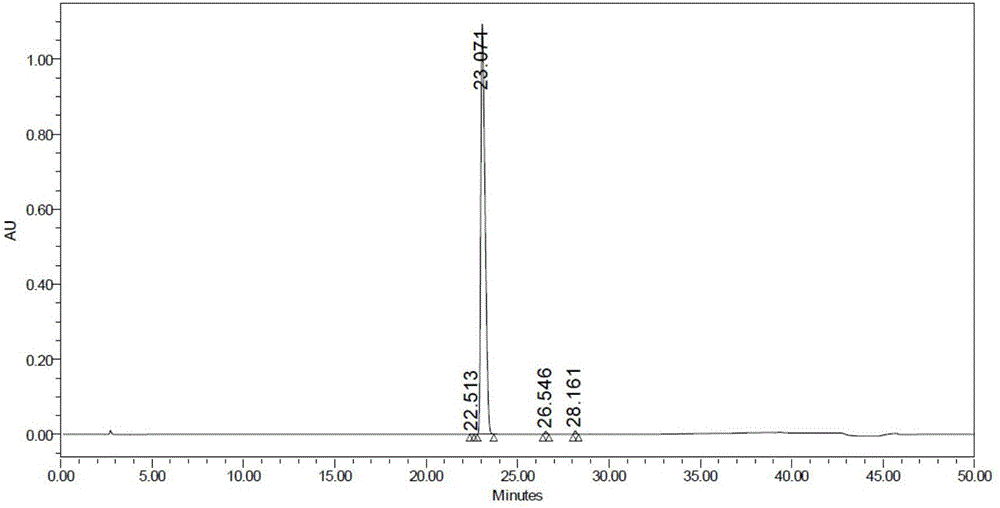
Image
Examples
Embodiment 1
[0093] Example 1 Preparation of Fmoc-Asp(OtBu)-FMK
[0094] 1.1 Fmoc-Asp(OtBu)-N 2 preparation of
[0095]
[0096] Fmoc-Asp(OtBu)-OH (1eq, 0.5mol, 206g) and nitrogen methylmorpholine (1.25eq, 0.625mol, 63.2g) were added to the reaction kettle, and dissolved in THF (2.0L), and the Hydrazine lowered the temperature of the system to about -10°C, slowly added isobutyl chloroformate (1.15eq, 0.575mol, 78.5g) dropwise, and kept at -10°C for 30 minutes. Then diazomethane-diethyl ether solution (4eq, 2.0mol, 84g) was slowly added to the above reaction system, after the addition was completed, the temperature was naturally raised to 25°C for 3 hours, and the reaction was detected by TLC spotting.
[0097] After the reaction was completed, 3.0 L of water and 0.1 L of acetic acid were slowly added to quench, and then 6.0 L of ethyl acetate was added for extraction. The separated organic phase was washed successively with 3.0 L saturated brine and 5% aqueous sodium bicarbonate solu...
Embodiment 2
[0106] The removal of tBu in the embodiment 2 Fmoc-Asp(OtBu)-FMK
[0107] 2.1 Preparation of Fmoc-Asp-FMK
[0108]
[0109] Cool 30% formic acid / DCM to 0°C, add Fmoc-Asp(OtBu)-FMK (1eq, 0.282mol, 100g) prepared according to the method of Example 1, stir to dissolve, naturally heat up to 25°C, and stir for 10 After 1 hour, TLC spot plate monitoring.
[0110] After the reaction was completed, the solvent was removed by a rotary evaporator, and 3.0 L of ethyl acetate was added for extraction. The separated organic phase was washed successively with 3.0 L saturated brine and 5% aqueous sodium bicarbonate solution, and finally dried with anhydrous magnesium sulfate. The solvent was removed by a rotary evaporator, recrystallized with absolute ethanol, and dried to obtain 94.4 g of white solid Fmoc-Asp-FMK, with a yield of 90.2%.
[0111] 2.2 Preparation of Fmoc-Asp-FMK
[0112]
[0113] Cool 50% TFA / DCM to 0°C, and add Fmoc-Asp(OtBu)-FMK (1eq, 0.282mol, 100g) prepared acco...
Embodiment 3
[0127] Example 3 Coupling of Fmoc-Asp-FMK and 2-CTC resin
[0128]
[0129] 2-CTC resin (1.1eq, 296mmol, 269g) with a degree of substitution of 1.1mmol / g was added to a solid-phase reaction column, washed twice with DMF, swelled for 1 hour, and drained. Dissolve Fmoc-Asp-FMK (1.0eq, 269mmol, 100g) prepared according to 2.4 in Example 2 in 700ml DMF and cool down to 2-5°C, add DIPEA (3.3eq, 888mmol, 115g), keep stirring for 10 minutes After that, it was added to the above-mentioned solid-phase reaction column, stirred with nitrogen gas for 1 hour, then added with 200ml of methanol and stirred for 30 minutes, then washed with DMF for 4 times, and the DMF was drained for later use to obtain Fmoc-Asp-FMK-CTC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com