A kind of preparation method of fluoromethyl ketone peptide series compound
A compound and carboxyl technology, applied in the field of compound preparation, can solve problems such as unfavorable industrial production, limited industrial production, and bad odor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
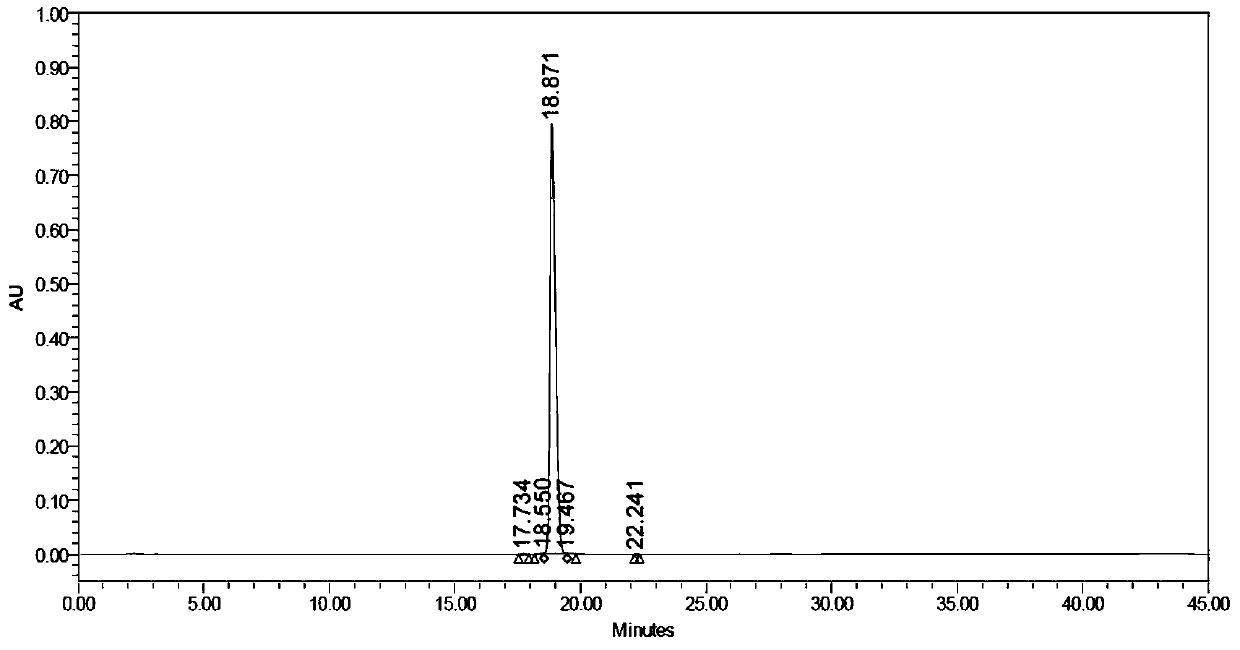
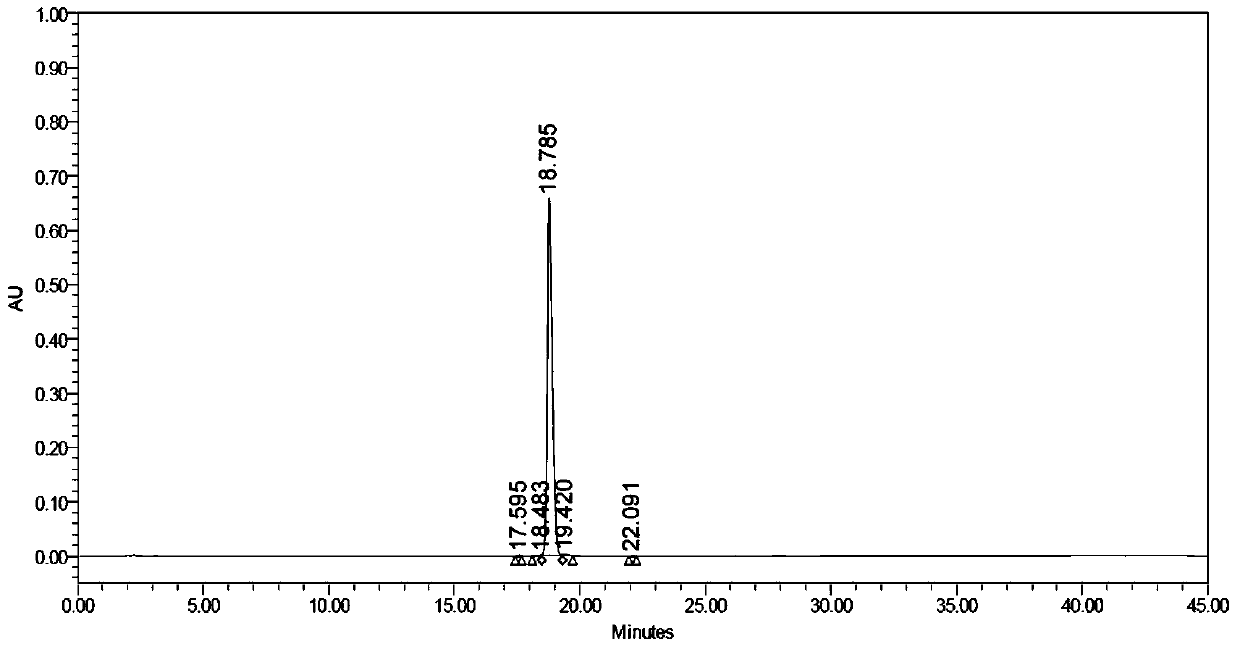
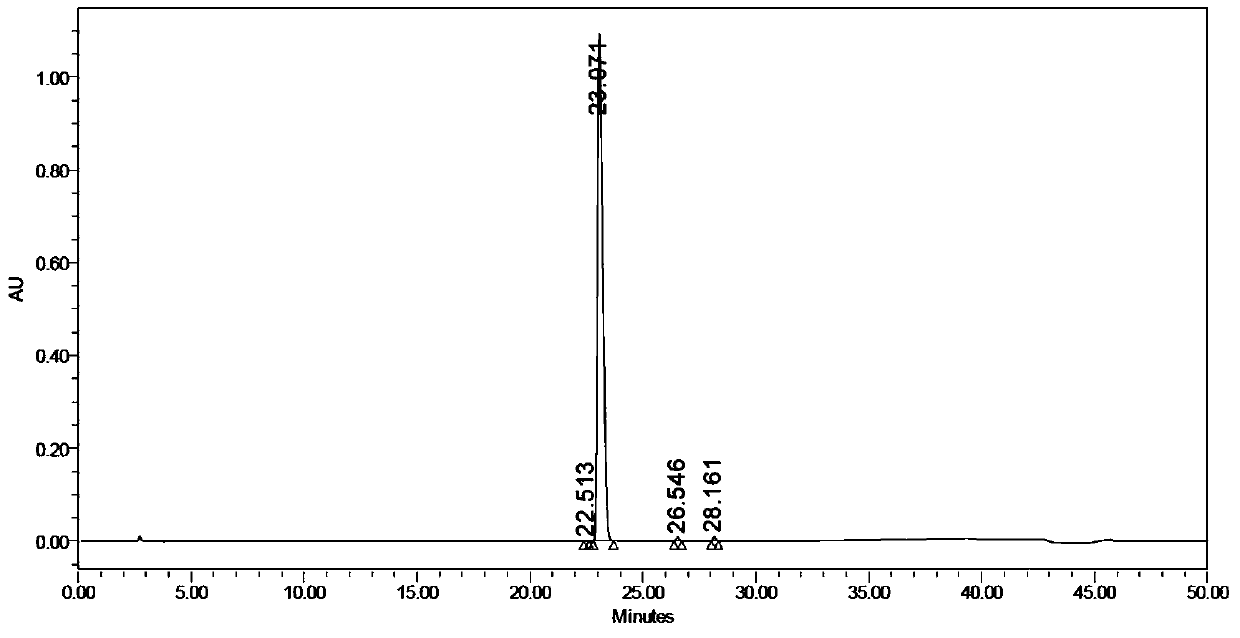
Image
Examples
Embodiment 1
[0093] The preparation of embodiment 1Fmoc-Asp (OtBu)-FMK
[0094] 1.1 Fmoc-Asp(OtBu)-N 2 preparation of
[0095]
[0096] Fmoc-Asp(OtBu)-OH (1eq, 0.5mol, 206g) and nitrogen methylmorpholine (1.25eq, 0.625mol, 63.2g) were added to the reaction kettle, and dissolved in THF (2.0L), and the Hydrazine lowered the temperature of the system to about -10°C, slowly added isobutyl chloroformate (1.15eq, 0.575mol, 78.5g) dropwise, and kept at -10°C for 30 minutes. Then diazomethane-diethyl ether solution (4eq, 2.0mol, 84g) was slowly added to the above reaction system, after the addition was completed, the temperature was naturally raised to 25°C for 3 hours, and the reaction was detected by TLC spotting.
[0097] After the reaction was completed, 3.0 L of water and 0.1 L of acetic acid were slowly added to quench, and then 6.0 L of ethyl acetate was added for extraction. The separated organic phase was washed successively with 3.0 L saturated brine and 5% aqueous sodium bicarbona...
Embodiment 2
[0106] The removal of tBu in the embodiment 2Fmoc-Asp(OtBu)-FMK
[0107] 2.1 Preparation of Fmoc-Asp-FMK
[0108]
[0109] Cool 30% formic acid / DCM to 0°C, add Fmoc-Asp(OtBu)-FMK (1eq, 0.282mol, 100g) prepared according to the method of Example 1, stir to dissolve, naturally heat up to 25°C, and stir for 10 After 1 hour, TLC spot plate monitoring.
[0110] After the reaction was completed, the solvent was removed by a rotary evaporator, and 3.0 L of ethyl acetate was added for extraction. The separated organic phase was washed successively with 3.0 L saturated brine and 5% aqueous sodium bicarbonate solution, and finally dried with anhydrous magnesium sulfate. The solvent was removed by a rotary evaporator, recrystallized with absolute ethanol, and dried to obtain 94.4 g of white solid Fmoc-Asp-FMK, with a yield of 90.2%.
[0111] 2.2 Preparation of Fmoc-Asp-FMK
[0112]
[0113] Cool 50% TFA / DCM to 0°C, and add Fmoc-Asp(OtBu)-FMK (1eq, 0.282mol, 100g) prepared accor...
Embodiment 3
[0127] The coupling of embodiment 3Fmoc-Asp-FMK and 2-CTC resin
[0128]
[0129] 2-CTC resin (1.1eq, 296mmol, 269g) with a degree of substitution of 1.1mmol / g was added to a solid-phase reaction column, washed twice with DMF, swelled for 1 hour, and drained. Dissolve Fmoc-Asp-FMK (1.0eq, 269mmol, 100g) prepared according to 2.4 in Example 2 in 700ml DMF and cool down to 2-5°C, add DIPEA (3.3eq, 888mmol, 115g), keep stirring for 10 minutes After that, it was added to the above-mentioned solid-phase reaction column, stirred with nitrogen gas for 1 hour, then added with 200ml of methanol and stirred for 30 minutes, then washed with DMF for 4 times, and the DMF was drained for later use to obtain Fmoc-Asp-FMK-CTC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com