Conjugates and their preparation and use
A conjugate and application technology, applied in the fields of life science and chemical biology, can solve the problems of difficult to predict the quantitative relationship of antibody/drug coupling, poor tissue penetration ability, product heterogeneity, etc., so that it is not easy to cause immune response , Strong tissue penetration ability, small molecular weight effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0087] Preparation method of the conjugate
[0088] According to another aspect of the present invention, the present invention also provides a method for preparing the aforementioned conjugate. According to an embodiment of the invention, the method includes:
[0089] (1) Synthesize the targeting peptide by solid-phase peptide synthesis, so as to obtain the resin bonded with the targeting peptide, wherein the targeting peptide has the amino acid sequence IHGHHIISVG, and use Fmoc in the solid-phase peptide synthesis - Glycine-Wang resin, the glycine bonding amount of the Fmoc-glycine-Wang resin is 0.4mmol / g as a starting material, and N,N-dimethylformamide containing 20 volume% hexahydropyridine is used for deprotection ( DMF) solution, deprotection 2 times, each 5min, the coupling step uses 3 times the molar amount of Fmoc-amino acid and 3 times the molar amount of O-benzotriazole-tetramethyluronium hexafluorophosphate (HBTU), N-methylmorpholine (NMM) as a basic catalyst; ...
Embodiment 1
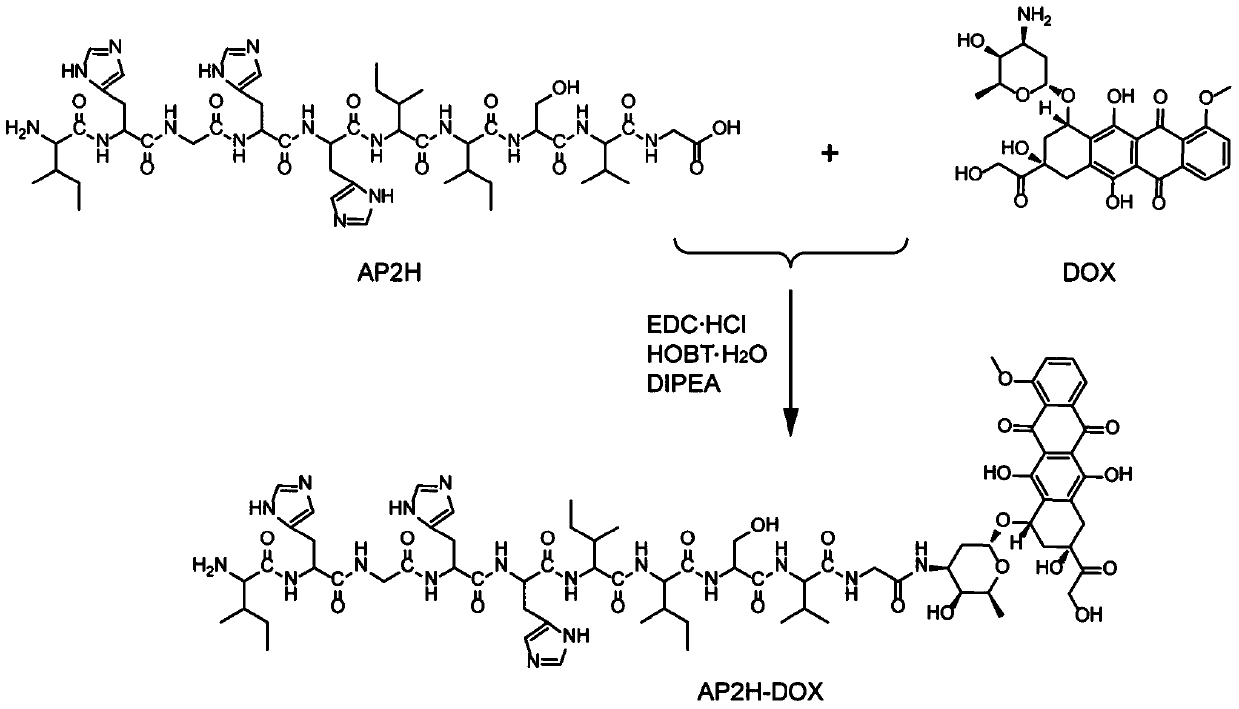
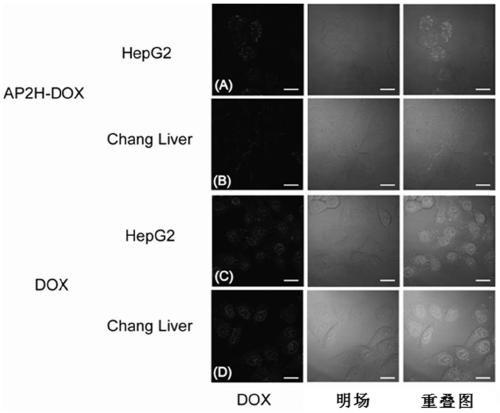
[0100] Example 1 Synthesis and Performance Investigation of Amide Bond-Coupled Polypeptide Drug Conjugate-1 (AP2H-DOX)
[0101] (1) Design and synthesis of AP2H-DOX
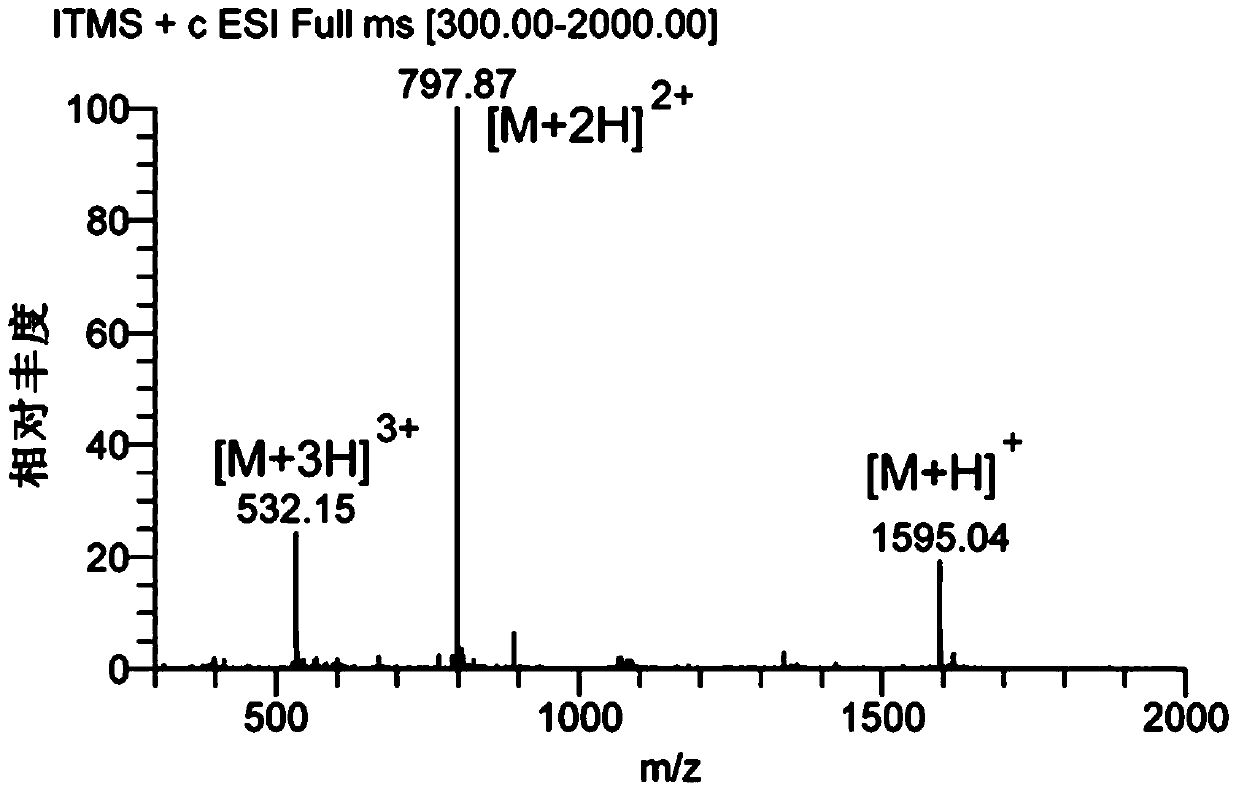
[0102] Amino acids form polypeptides through the condensation of amino groups and carboxyl groups. Based on this, the inventors first simulated the chemical bond-amide bond that forms the polypeptide, and condensed the carboxyl group at the C-terminal of the AP2H polypeptide with the amino group of the anticancer drug doxorubicin to form an amide bond to form a polypeptide drug. Conjugate-1 (AP2H-DOX), the synthesis process is as follows figure 1 . Dissolve AP2H polypeptide (1eq) in DMF, add DOX·HCl (2eq) to it, then add DIPEA (10eq), EDC·HCl (3eq), HOBt·H 2 O(3eq), react at room temperature for 6h. The reaction solution was purified by a semi-preparative liquid chromatography column to obtain the final product AP2H-DOX. After purification by liquid chromatography, the product was identified using ESI mass sp...
Embodiment 2
[0107] Example 2 Synthesis and Performance Investigation of Redox Responsive Polypeptide Drug Conjugate-2 (AP2H-s-s-DOX)
[0108] (1) Design and synthesis of AP2H-s-s-DOX
[0109] Using the commercial reagent 3,3-dithiodipropionate (N-succinimide) ester (DSP) to couple AP2H to DOX, the peptide drug conjugate-2 (AP2H-s-s-DOX) The synthesis process and the principle of GSH-responsive drug release such as Figure 5 shown. Specifically, AP2H polypeptide (1eq) was dissolved in DMSO, DOX·HCl (1eq) was added, after mixing evenly, DSP (1eq) was added, and then Et 3 N (6eq), react at room temperature for 12h. The reaction solution was purified by a semi-preparative liquid chromatography column to obtain the final product AP2H-s-s-DOX. After purification of the reaction target by liquid chromatography, the product was identified by ESI mass spectrometry, it can be seen that [M+H] + :1787.01,[M+2H] 2+ :894.05,[M+3H] 3+ :596.24 and other mass spectrum signals, indicating that the t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com