Amorphism of compound A benzoate, preparation method thereof, and amorphism-containing medicinal composition
A technology of benzoate and benzoate chemistry, applied to the amorphous form of compound A benzoate and its preparation, and the field of pharmaceutical compositions containing the amorphous form, which can solve the problem of lack of hydrophobic transmembrane domains and other problems , to achieve the effect of being conducive to preparation and use, simple method and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 compound A
[0041] According to the methods of Examples 2 and 3 of WO2011079778 specification, compound A was prepared using the following technical synthesis route:
[0042] The obtained compound A, 1 H-NMR (400MHz, DMSO, ppm): δ7.96(m,1H),7.36(br,1H),7.29(d,1H),5.23(s,2H),3.15(m,3H),2.72( m,2H),2.23(s,3H),1.78(d,1H),1.64(d,1H),1.47(m,1H),1.12(m,1H).MS: m / z,343(100% ,M+1).
[0043]
[0044] Concrete preparation steps are as follows:
[0045] Step A. 1-Bromo-4-fluoro-2-(methylisothiocyanate)benzene (2)
[0046]To a solution of 1-bromo-2-(bromomethyl)-4-fluorobenzene (1,5.36 g, 20.0 mmol) in DMF (20 mL) was added sodium iodide (1.20 g, 8.00 mmol) and potassium thiocyanate (3.88g, 40.0mmol). The mixture was heated to 80° C. for 12 h under a nitrogen atmosphere, cooled to room temperature, 100 mL of water was added thereto, and extracted with ethyl acetate (50 mL×2), the combined organic layers were washed with saturated brine, a...
Embodiment 2
[0062] The preparation of embodiment 2 compound A benzoate
[0063] Prepare 95% ethanol solution: add 228mL ethanol to a 500mL beaker, add 12mL water, stir evenly, set aside.
[0064] Take 2.14g of benzoic acid, add 10mL of 95% ethanol at room temperature and stir to dissolve, and set aside; add 60g of compound A refined product and 120mL of 95% ethanol to a 500mL reaction bottle, stir, dissolve, filter, and wash with 18ml of 95% ethanol; The ethanol solution of benzoic acid was added dropwise at 15 °C. After the dropwise addition was completed, the mixture was washed with 95% ethanol and dried under reduced pressure to constant weight to obtain 42.4 g of compound A benzoate.
[0065] 1 HNMR (DMSO-d 6 ,400MHz,ppm)δ7.96-7.88(m,3H),7.45-7.26(m,5H),6.80(brs,3H),5.21(q,2H),3.41(d,1H),3.11-3.08( m,2H),2.91-2.79(m,2H),2.23(s,3H),1.95-1.91(m,1H),1.78-1.74(m,1H),1.57-1.42(m,2H).MS: m / z,341(100%,M-1),343(100%,M+1).
Embodiment 3
[0066] The amorphous preparation of embodiment 3 compound A benzoate
[0067] Put 5.0 g of compound A benzoate in 25 mL of dichloromethane solvent, stir to dissolve, and concentrate to dryness at 20°C to 30°C to obtain a bubble-like product, which is vacuum-dried at room temperature to prepare an amorphous form of compound A benzoate .
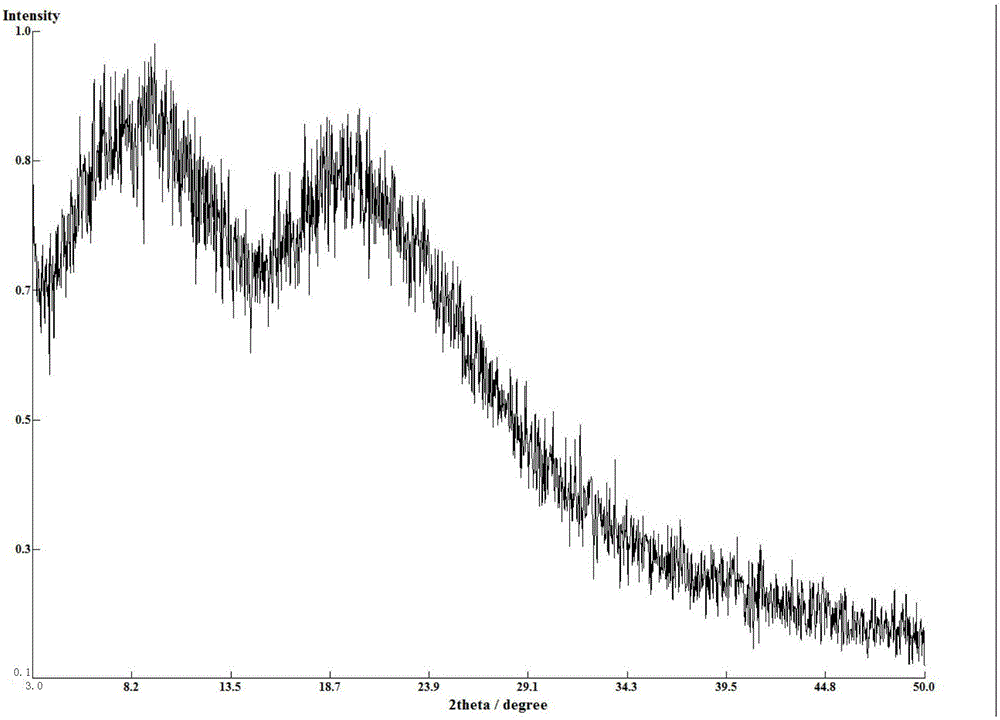
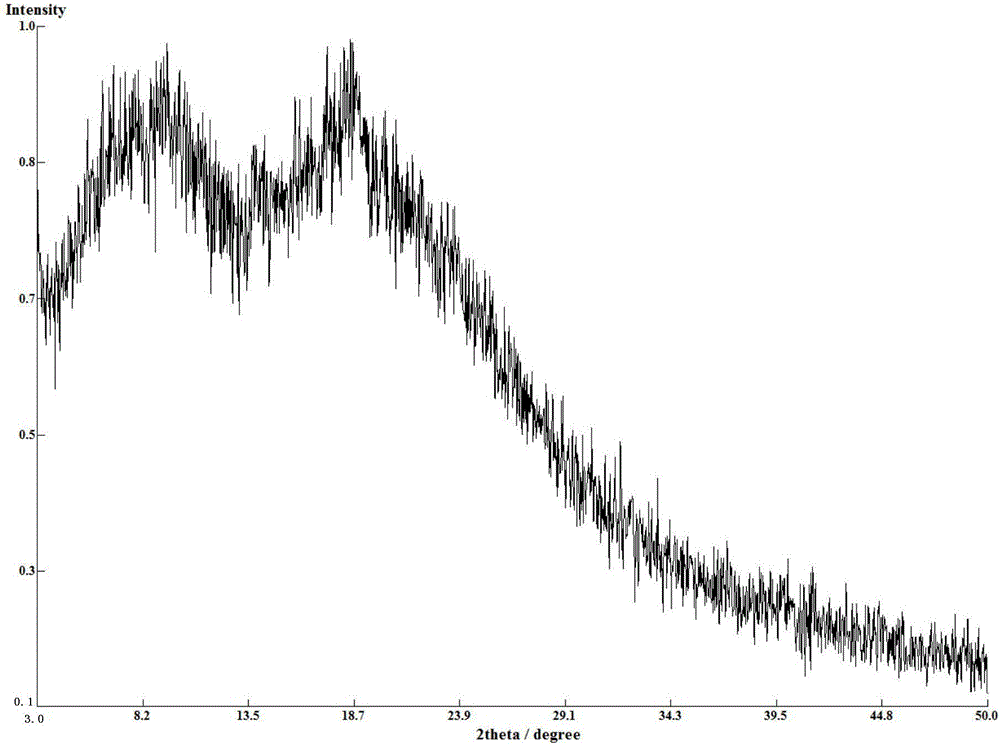
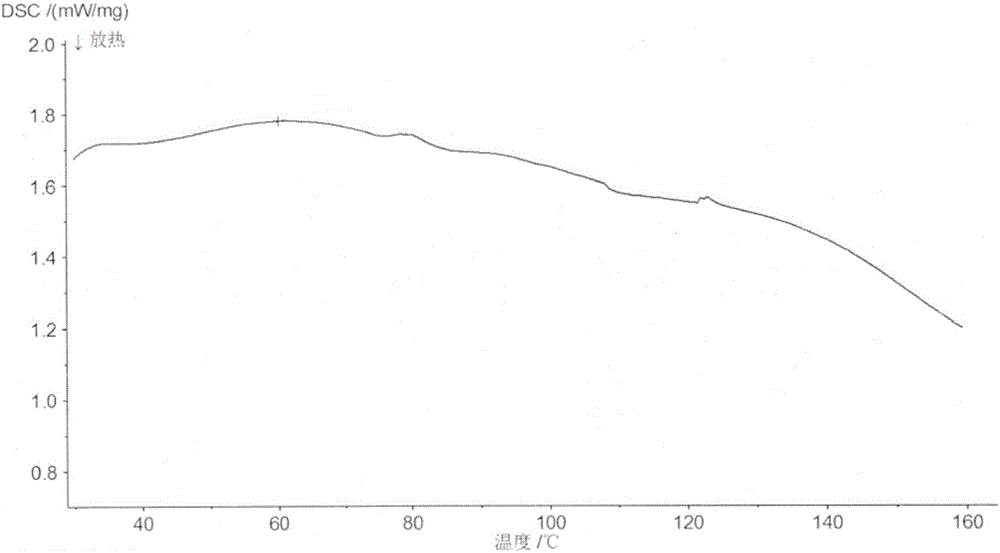
[0068] The amorphous X-ray diffraction pattern of gained compound A benzoate is as figure 1 As shown, the DSC spectrum is as image 3 As shown, the TG spectrum is as Figure 5 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com