Gliquidone compound preparation capable of reducing renal damage
A technology of glibaquinone and compound preparations, which is applied in the field of diabetes pharmaceutical preparations, can solve the problems that the quality of life of patients treated with the disease is not very good, the protection of kidneys and blood vessels is not obvious, and the body develops drug resistance, etc. The effect of quality of life, avoiding the decline of filtration capacity, and reducing kidney damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 gliquidone compound capsule
[0033] Weigh the dried Panax notoginseng root, put it into the extraction container after crushing, pour 70% (v / v) ethanol into it according to the ratio of material to liquid (mass) 5:4, as a desorbent, soak the Panax notoginseng powder, and desorb at room temperature for 30 minutes . Then pour 8 times the amount of distilled water with a temperature of 45°C into the fully soaked medicinal materials, and quickly reduce the pressure to -0.084MPa, so that small bubbles will emerge from the inside of the Panax notoginseng powder, and achieve internal boiling and extraction for 4 minutes, then vacuum filter. Get the extracted filter residue (i.e. medicinal residue), carry out the second extraction according to the same conditions as above-mentioned first extraction, only change the amount of distilled water into 5 times of the filter residue quality. The two extracts of Panax notoginseng were combined, concentr...
Embodiment 2
[0038] Embodiment 2 GLiquidone Compound Capsule Drug Efficacy Detection
[0039] 1. DN model copy
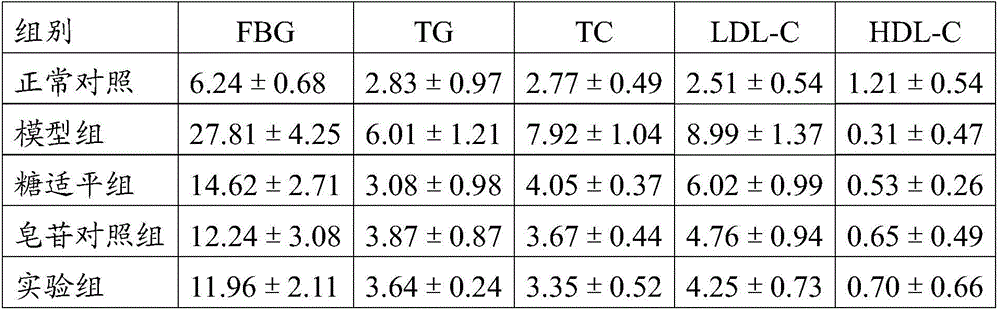
[0040] SPF grade male SD rats were selected as experimental animals, fed a high-energy diet (high-sugar and high-fat diet) supplemented with a small dose of intraperitoneal injection of streptozotocin, induced non-insulin-dependent (type 2) diabetes, and detected blood sugar ≥ When it is above 16.7mmol / L, it is determined that the diabetic rat model is successfully established. The diabetic rat model progressed to diabetic nephropathy (DN) according to the natural course of the disease, and the fasting blood glucose was detected to be ≥16.7mmol / L, the urinary protein excretion rate was >20μg / min, and the urine volume was 150% of the normal amount, which was determined to be the replication of the diabetic nephropathy rat model success.
[0041] 2. Experimental medication and group feeding
[0042] Referring to the literature "Observation on the Curative Effect of GLiquidone H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com