Preparation method and application of physiological pH (potential of hydrogen) sensing dinuclear ruthenium complex
A technology of ruthenium complexes and bridging ligands is applied in the fields of fluorescent sensors and bio-inorganic chemistry, which can solve the problems of rare application of ruthenium-based metal complexes, and achieve the effects of stable structure and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment one, the preparation of ruthenium complex
[0026] 1.1 Preparation of Ligand HL:
[0027] Ligand HL was prepared according to the route shown in the following formula:
[0028]
[0029] Add 150mg pyrazino[2,3-f][1,10]phenanthroline-2-carboxylic acid, 112mg 1,10-phenanthroline-5,6-diamine, and 22g polyphosphoric acid into 25mL three ports Bottle, nitrogen protection, mechanical stirring to mix evenly. Heating to 120°C first, then raising the temperature to 190°C for 7 hours, the mixture changed from red transparent to dark green. Cool to room temperature under the protection of nitrogen, and pour the obtained viscous liquid into ice water to obtain a light yellow-green precipitate, which is neutralized with ammonia water, and the color of the precipitate deepens. The precipitate was collected by suction filtration, washed with water and Soxhlet extraction with methanol, and vacuum dried to obtain 139 mg (57.4%) of HL as a solid. Infrared spectrum (KBr, ...
Embodiment 2
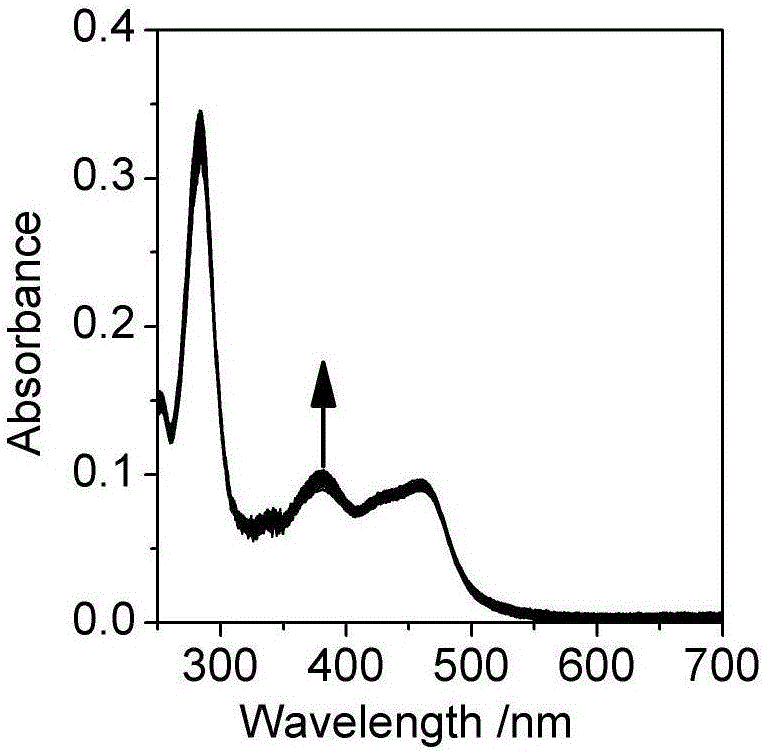
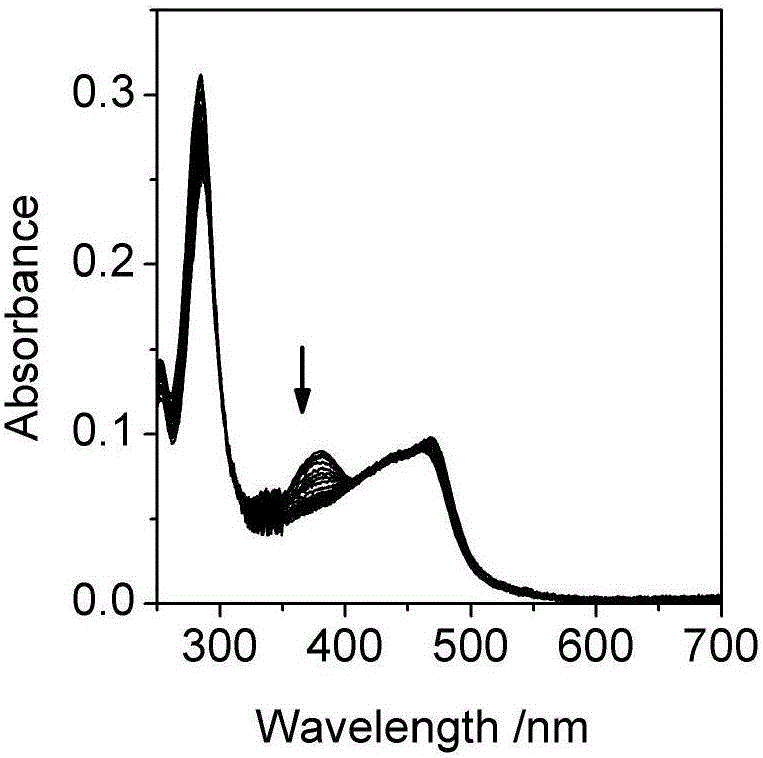
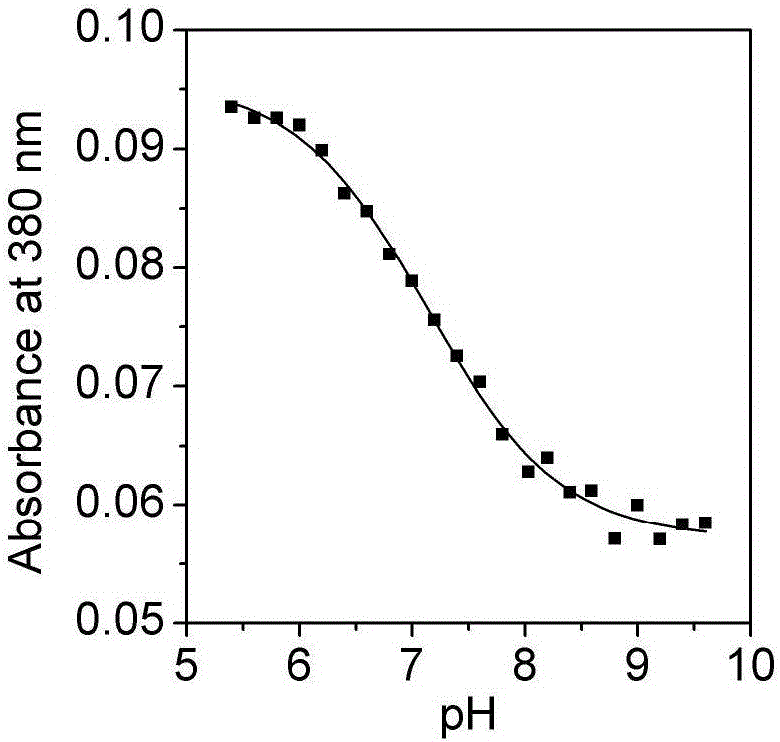
[0032] Embodiment two, the influence of pH change on the ultraviolet-visible absorption spectrum and photoluminescence spectrum of the Britton-Roberson (BR) buffer solution of ruthenium complex
[0033] The ultraviolet-visible absorption spectrum was measured on a UV-2600 ultraviolet-visible spectrophotometer, and the BR buffer solution was used as a reference solution during the measurement. Fluorescence emission spectra were measured on a Cary Eclipse fluorescence spectrophotometer. The quantum efficiency of luminescence is obtained by ruthenium terpyridine [Ru(bpy) 3 ] 2+ as a standard (Φ std =0.028), the measured concentration is 1.0×10 -6 mol / L [Ru(bpy) 3 ] 2+ The UV-visible absorption spectrum and emission spectrum of the aqueous solution, read the absorbance A at 468nm of the UV-visible absorption spectrum std and the integrated intensity I of the emission spectrum std , according to formula (1):
[0034] Φ=Φ std (A std / A)(I / I std ) (1)
[0035] Φ and Φ st...
Embodiment 3
[0037] Embodiment three, the mensuration of unknown water sample pH
[0038] Take a certain amount of unknown water sample, add sodium chloride to it to a concentration of 0.1M, and add a quantitative complex to make the concentration 2μM, measure the ultraviolet absorption and emission spectra of the water sample, and calculate the According to the standard curve (Fig. 2(c)) for the luminescence quantum yield, read the pH corresponding to the quantum yield, so as to know the pH of the unknown water sample.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com