Quality detection method of ginseng basis-consolidating oral solution
A technology of oral liquid and ginseng, which is applied in the field of quality inspection of ginseng guben oral liquid, can solve the problems of not being able to fully control the quality of ginseng guben oral liquid, not enough to represent ten medicinal materials, poor accuracy and controllability, etc. Achieve scientific and reasonable detection methods, meet medical and market needs, and ensure stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
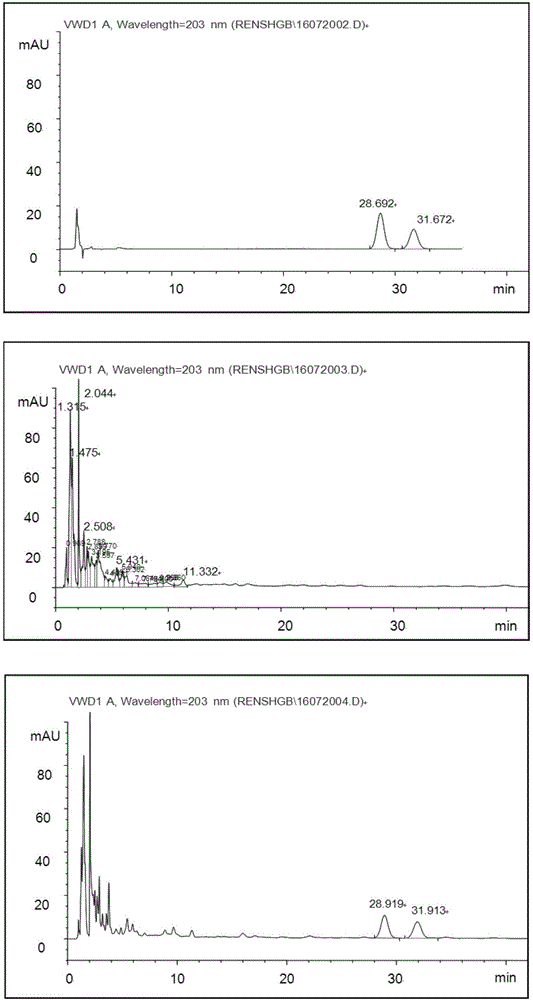
[0050] The HPLC methodology research of embodiment 1 ginsenoside Rg1, ginsenoside Re
[0051] 1) Instruments and reagents: Agilent 1100 high performance liquid chromatograph, VWD detector; acetonitrile and methanol are chromatographically pure, other reagents are analytically pure, and water is double distilled water.
[0052] 2) Chromatographic conditions: Kromasil C 18 Chromatographic column (4.6mm×150mm, 5μm); mobile phase is acetonitrile-water (19:81); detection wavelength is 203nm; flow rate is 1mL / min, column temperature is 25°C.
[0053] 3) Preparation of the test solution: Accurately measure 50mL of this product, concentrate to 25mL, let it cool, put it in a separatory funnel, add ethyl acetate to shake and extract twice (30mL, 20mL), discard the ethyl acetate layer , the water layer was shaken and extracted 4 times with water-saturated n-butanol, 20mL each time, combined the n-butanol extracts, washed 3 times with 5% sodium hydroxide solution, 30mL each time, discard...
Embodiment 2
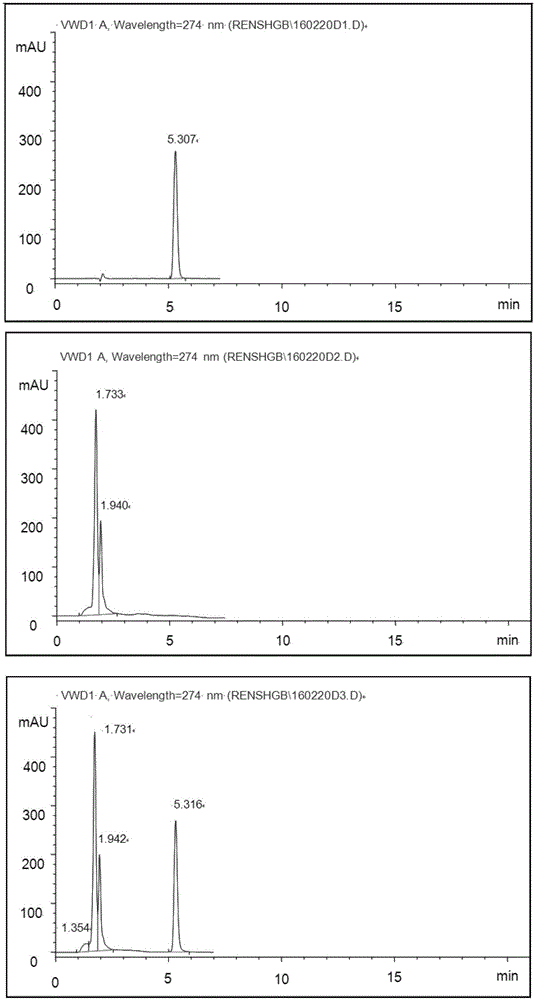
[0088] The HPLC methodology research of embodiment 2 paeonol
[0089] 1) Instruments and reagents: SHMADZU high-performance liquid chromatography, SPD-10A ultraviolet detector. Methanol is chromatographically pure, other reagents are analytically pure, and water is double distilled water.
[0090]2) Chromatographic conditions: HYPER SIL BDS C 18 5μm column; the mobile phase is methanol: water = 60:40; the detection wavelength is 274nm; the flow rate is 0.8mL / min.
[0091] 3) Preparation of the test solution: draw 3mL of this product, put it in a 25mL volumetric flask, add absolute ethanol to dilute to the mark, shake well, and centrifuge. Take the supernatant and filter it with a 0.45 μm microporous membrane to obtain the obtained solution.
[0092] 4) Preparation of reference substance solution: Accurately weigh an appropriate amount of paeonol reference substance (Paeonol reference substance batch number: 110708-200506, China Institute for the Control of Pharmaceutical an...
Embodiment 3
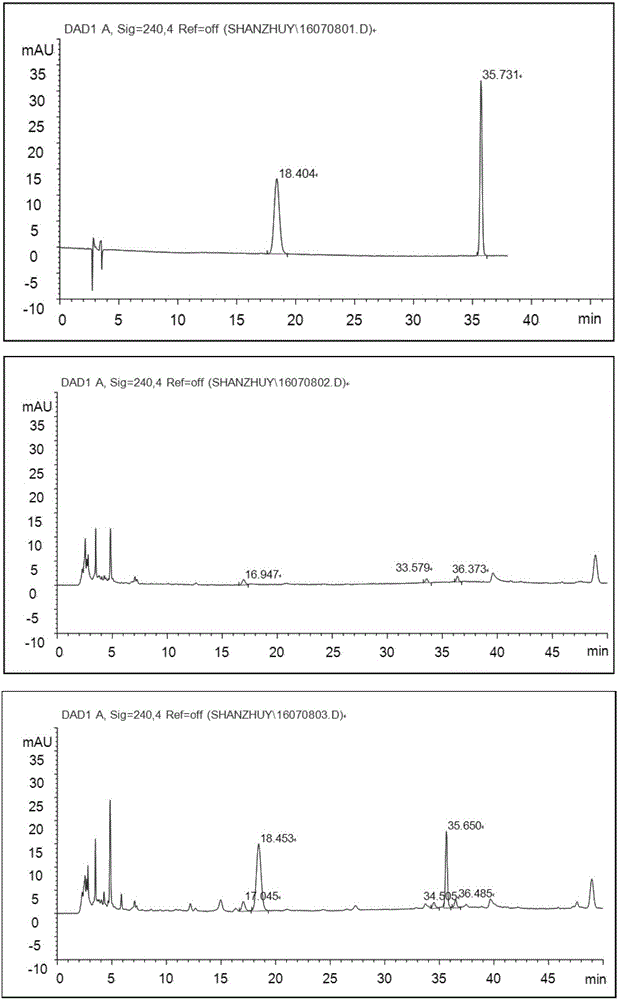
[0128] The HPLC methodology research of embodiment 3 morroniside, loganin
[0129] 1) Instruments and reagents: Agilent 1100 high performance liquid chromatography, DAD detector. Acetonitrile and methanol are chromatographically pure, other reagents are analytically pure, and water is double distilled water.
[0130] 2) Chromatographic conditions: Kromasil C 18 Chromatographic column (4.6mm×250mm, 5μm); mobile phase: use acetonitrile as mobile phase A, 0.3% phosphoric acid solution as mobile phase B, carry out gradient elution as specified in the table below;
[0131]
[0132] The detection wavelength is 240nm; the column temperature is 35°C; the flow rate is 1mL / min.
[0133] 3) Preparation of the test solution: Accurately measure 5mL of this product under the difference in filling volume, put it in a 50mL measuring bottle, add 50% methanol to dilute to the mark, shake well, centrifuge, take the supernatant and use a microporous filter membrane (0.45μm) filter, that is....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com