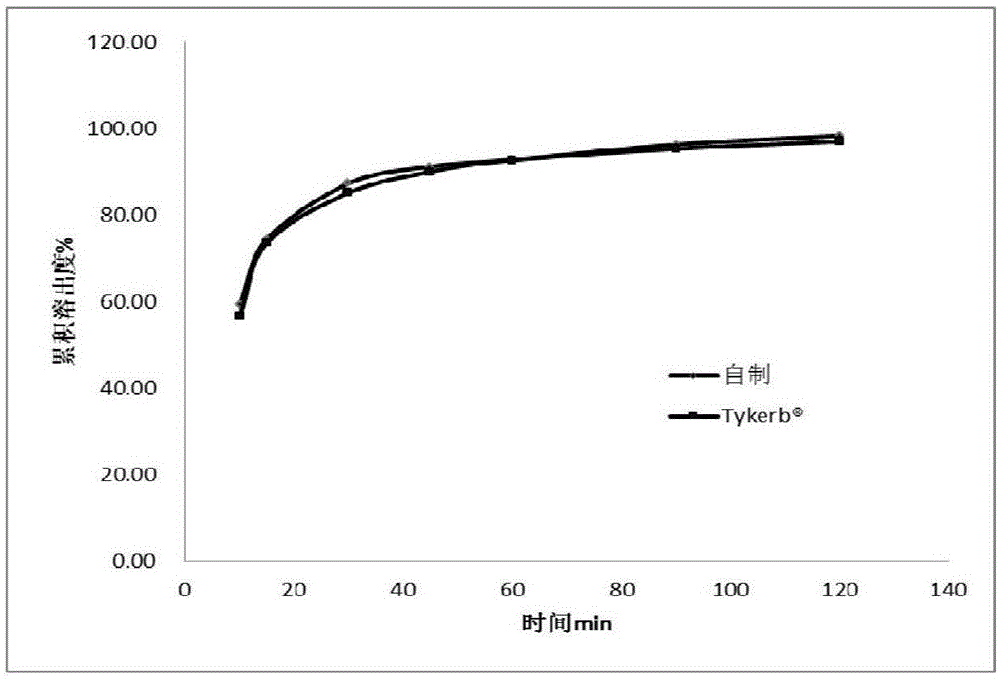
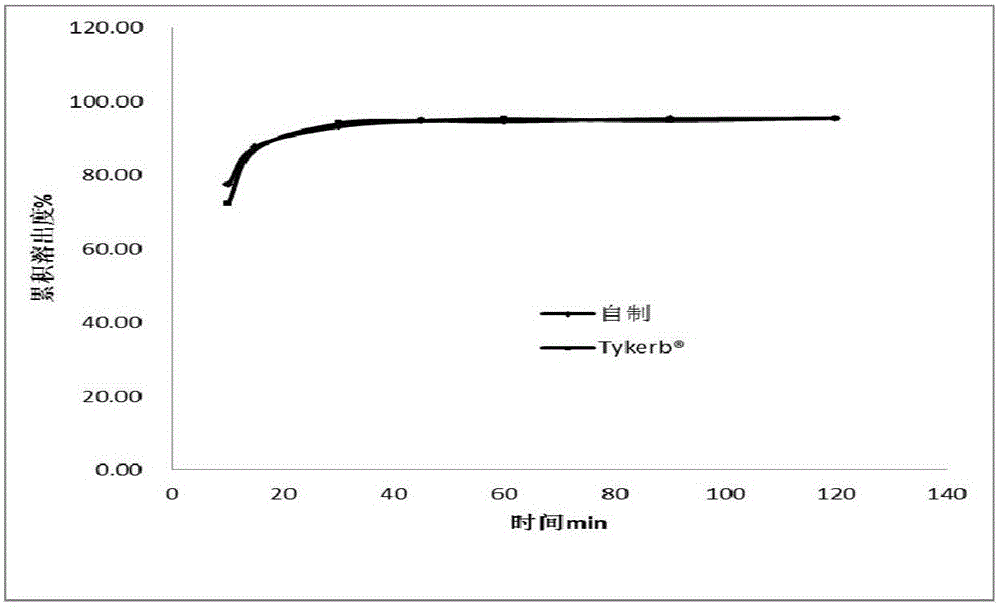
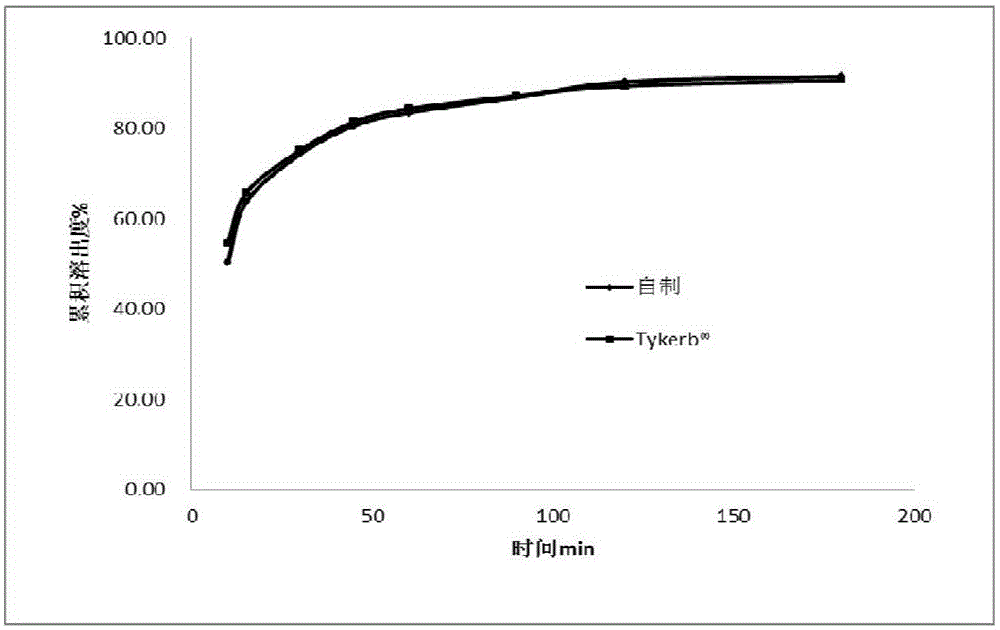
Lapatinib ditosylate tablet and preparation method thereof
A technology for lapatinib xylene sulfonate and patinib tablets, which is applied in the directions of pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve unfavorable product dissolution and sample flocculation. , poor solubility and other problems, to achieve the effect of improving the accumulation of agglomeration, ensuring stability, and the preparation process being mature and stable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation of embodiment 1 lapatinib ditosylate tablet of the present invention
[0039] The lapatinib ditosylate tablet of the present invention comprises the following compositions by weight:
[0040]
[0041]Preparation: The dosage form can be produced using conventional tablet pharmaceutical equipment, and the specific preparation method is as follows: initially mix the raw material lapatinib ditosylate and colloidal silicon dioxide, pass through a 100-mesh sieve, and mix evenly. Microcrystalline cellulose and mannitol were respectively passed through 100 mesh sieves, then weighed respectively with croscarmellose sodium and hypromellose according to the amount, and added to the above mixture in sequence, Mix under the wet granulator, the mixing speed is 300rpm, the cutter speed is 500rpm; then add purified water to prepare soft material, during the soft material production, the stirring speed is 300rpm, the cutter speed is 1200rpm, after granulation with 24 ...
Embodiment 2
[0048] Embodiment 2 The preparation of lapatinib ditosylate tablet of the present invention
[0049] The lapatinib ditosylate tablet of the present invention comprises the following compositions by weight:
[0050]
[0051] Preparation: The dosage form can be produced using conventional tablet pharmaceutical equipment, and the specific preparation method is as follows: pass the raw drug lapatinib ditosylate and colloidal silicon dioxide through a 150-mesh sieve, and mix well. Microcrystalline cellulose and mannitol were respectively passed through a 100-mesh sieve, and then added to the above-mentioned mixture in sequence with croscarmellose sodium and hydroxypropyl cellulose and mixed evenly, and mixed in a high-shear wet granulator. Mix at the bottom, the mixing speed is 200rpm, the cutter speed is 600rpm; then add purified water to prepare the soft material, during the soft material production, the stirring speed is 400rpm, the cutter speed is 1000rpm, and the 20-mesh si...
Embodiment 3
[0052] Embodiment 3 The preparation of lapatinib ditosylate tablet of the present invention
[0053] The lapatinib ditosylate tablet of the present invention comprises the following compositions by weight:
[0054]
[0055] Preparation: The dosage form can be produced using conventional tablet pharmaceutical equipment, and the specific preparation method is as follows: pass the raw drug lapatinib ditosylate and colloidal silicon dioxide through a 200-mesh sieve, and mix well. Microcrystalline cellulose KG802 and mannitol were passed through a 80-mesh sieve, and then added to the above-mentioned mixture in sequence with croscarmellose sodium and hypromellose, and mixed evenly under a high-shear wet granulator. The mixing speed is 400rpm, the cutter speed is 600rpm; then add purified water to prepare soft materials, the stirring speed is 400rpm, the cutter speed is 800rpm, granulate with a 16-mesh sieve and dry at 50°C until the water content is about 2%, after 24 After mesh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com