Large-scale preparation method for wurtzite Cu2ZnSnS4 nanocrystal
A technology of nanocrystals and wurtzite, applied in nanotechnology, chemical instruments and methods, inorganic chemistry, etc., can solve the inherent limitations of product yield, which is difficult to increase, unfavorable for high-efficiency, rapid preparation, and difficult to expand the scale of the reaction. The preparation process is intuitive and controllable, the photoelectric conversion efficiency is improved, and the cost is low
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
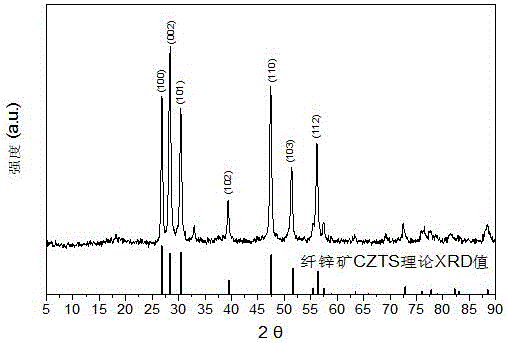
Embodiment 1
[0028] (1) Add 14mmol of cuprous iodide, 10mmol of zinc chloride and 8mmol of tin chloride pentahydrate into 48ml of oleylamine solvent, slowly heat the solution to 120°C under vacuum, and keep it warm for 30 minutes to completely remove the Water and oxygen are then cooled to room temperature to obtain a precursor solution;
[0029] (2) Add 16ml of n-dodecanemercaptan to the precursor solution to obtain a mixed solvent, then add 20mmol of carbon disulfide, heat to 140°C for 30 minutes, then heat to 300°C for 30 minutes, during the heating process, pass through a magnetic stirrer The reaction solution is stirred, and the stirring speed is 1000r / min;
[0030] (3) Stop heating, and after the reaction system is cooled to 60°C, add 30 ml each of n-hexane, methanol and ethanol to the reaction liquid at a time, centrifuge at 12000 r / min for 5 min, discard the supernatant, and repeat the above centrifugation operation for 3 times, the cleaned product was dried in a drying oven at 60...
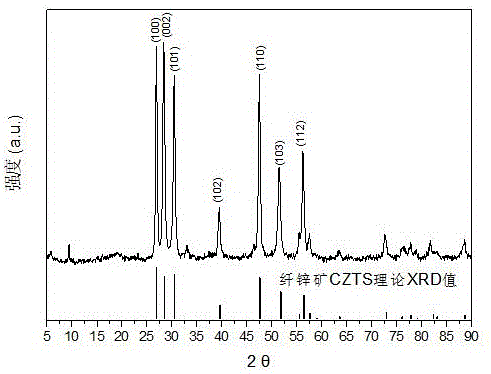
Embodiment 2
[0033] (1) Add 18mmol of cuprous iodide, 10mmol of zinc chloride and 5mmol of tin chloride pentahydrate into 48ml of oleylamine solvent, slowly heat the solution to 120°C under vacuum, keep it warm for 30 minutes, and completely remove the The water and oxygen are cooled to room temperature to obtain the precursor solution;
[0034] (2) Add 16ml of n-dodecanemercaptan to the precursor solution to obtain a mixed solvent, then add 30mmol of carbon disulfide, heat to 160°C for 50 minutes, then heat to 280°C for 45 minutes, during the heating process, use a magnetic stirrer to The reaction solution is stirred, and the stirring speed is 800r / min;
[0035] (3) Stop heating, and after the reaction system is cooled to 60°C, add 30 ml each of n-hexane, methanol and ethanol to the reaction liquid at a time, centrifuge at 11000 r / min for 5 min, discard the supernatant, and repeat the above centrifugation operation 3 times, the cleaned product was dried in a drying oven at 70°C for 10 h ...
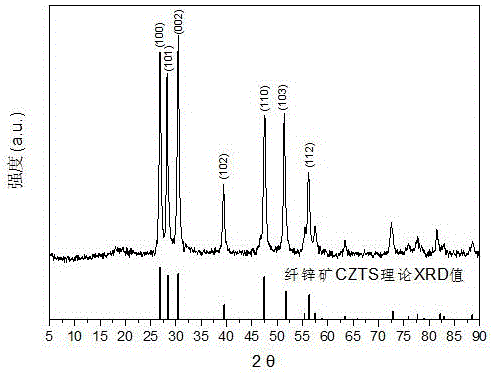
Embodiment 3
[0038](1) Add 20mmol of cuprous iodide, 10mmol of zinc chloride and 8mmol of tin chloride pentahydrate into 48 ml of oleylamine solvent, slowly heat the solution to 120°C under vacuum, keep it warm for 30 minutes, and completely remove the The water and oxygen are cooled to room temperature to obtain the precursor solution;
[0039] (2) Add 16ml of n-dodecanethiol to the precursor solution to obtain a mixed solvent, then add 40mmol of carbon disulfide, heat to 180°C for 20 minutes, and then heat to 250°C for 60 minutes. During the heating process, use a magnetic stirrer to The reaction solution is stirred, and the stirring speed is 700r / min;
[0040] (3) Stop heating, and after the reaction system is cooled to 60°C, add 30 ml each of n-hexane, methanol and ethanol to the reaction liquid at a time, centrifuge at 10,000 r / min for 5 min, discard the supernatant, and repeat the above centrifugation operation for 3 times, the cleaned product was dried in a drying oven at 80°C for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com