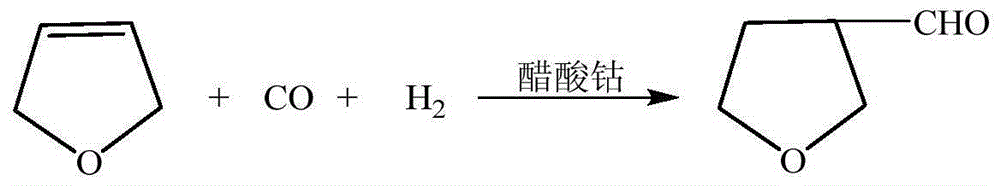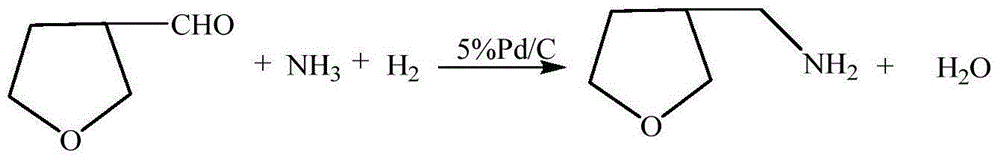3-methylamine tetrahydrofuran preparation method
A technology of methylamine tetrahydrofuran and tetrahydrofuran formaldehyde, which is applied in the field of preparation of 3-methylamine tetrahydrofuran, can solve the problems of not being suitable for industrial production and explosion risk, and achieve the effects of short reaction steps, less pollution of three wastes, and lower production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
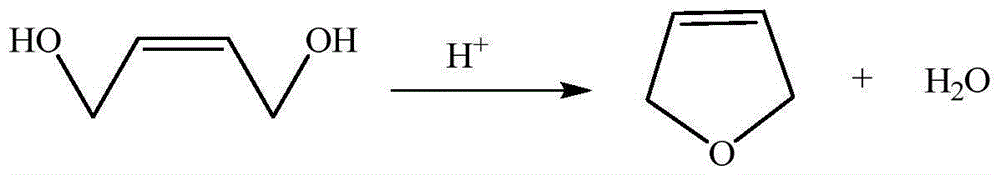
[0027] The preparation of embodiment 1 3-methylamine tetrahydrofuran
[0028] (1) Cyclization reaction to prepare 2,5-dihydrofuran
[0029] Weigh 1000kg 1,4-butenediol for use. Add 30kg of alumina and 100kg of 1,4-butenediol to the reaction kettle successively, stir and mix evenly, then raise the temperature to 130°C, then continue to add 900kg of 1,4-butenediol dropwise until the reaction is completed, and keep the temperature for 8 hours During the reaction process, the fraction below 102°C was continuously distilled, and the obtained fraction was left to stand for stratification. After the organic layer was dried and dehydrated, it was rectified at atmospheric pressure to collect the fraction at 64-67°C; the fraction at 64-67°C was collected in the water layer through distillation The desired 2,5-dihydrofuran was obtained by mixing the fractions at 64-67°C obtained twice, and a total of 787.5 kg of 2,5-dihydrofuran was obtained, with an HPLC purity of 95.03% and a yield of...
Embodiment 2-3
[0034] Example 2-3 Ring closure reaction to prepare 2,5-dihydrofuran
[0035] 2,5-dihydrofuran was prepared using the same operating method and the same type of raw materials as in step (1) of Example 1, the difference being the feed ratio and reaction time. The experimental results obtained are shown in Table 1:
[0036] Table 1:
[0037]
Embodiment 4-5
[0038] Embodiment 4-5 Formylation reaction prepares 3-THF
[0039] Adopt the operation method identical with embodiment 1 step (2) and same raw material to prepare 3-tetrahydrofurfuran formaldehyde, difference is feed ratio, water gas pressure, reaction temperature and reaction time, gained experimental result is as shown in table 2:
[0040] Table 2:
[0041]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com