Recombinant human fibroblast growth factor 21 fusion protein and application thereof in preparation of medicine for treating metabolic diseases
A technology of human fibroblasts and fusion proteins, applied in the fields of fibroblast growth factors and recombinant proteins, can solve the problems of increasing the difficulty of purification, the risk of immune reactions, etc., and achieve good blood sugar fluctuations and good hypoglycemic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Construction of hFGF21-HSA, HSA-hFGF21, hFGF21-3DHSA and 3DHSA-hFGF21 expression vectors
[0027] According to the codon preference of Escherichia coli, 4 genes were designed, and their nucleotide sequences are shown in the sequence listing as SEQ ID NO:1 (hFGF21-HSA), SEQ ID NO:2 (HSA-hFGF21), SEQ ID NO:3 (hFGF21-3DHSA) and SEQ ID NO: 4 (3DHSA-hFGF21). These four genes were sent to Shanghai Jierui Biological Co., Ltd. for synthesis, and NdeI and BamHI restriction sites were designed at both ends of each gene.
[0028] The four synthetic vectors and pET30a(+) containing their respective target gene fragments were double-enzyme-digested with NdeI and Bam HI respectively. Using T4 DNA ligase, the four target fragments were respectively ligated with the prokaryotic expression vector pET30a(+), the ligation reaction system was 10 µL, mixed well, ligated overnight at 4°C, and then transformed into E. coli DH5α respectively. The positive clones were picked and identified by...
Embodiment 2
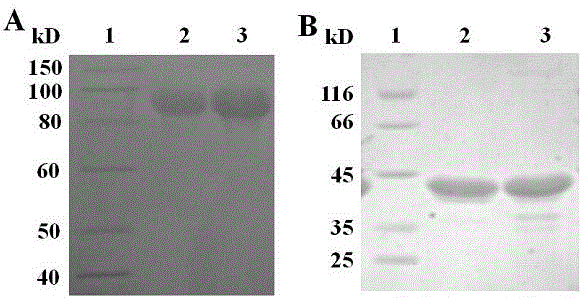
[0030] Expression and purification of four fusion proteins hFGF21-HSA, HSA-hFGF21, hFGF21-3DHSA and 3DHSA-hFGF21
[0031] (1) Transformation, culture and induction of expression
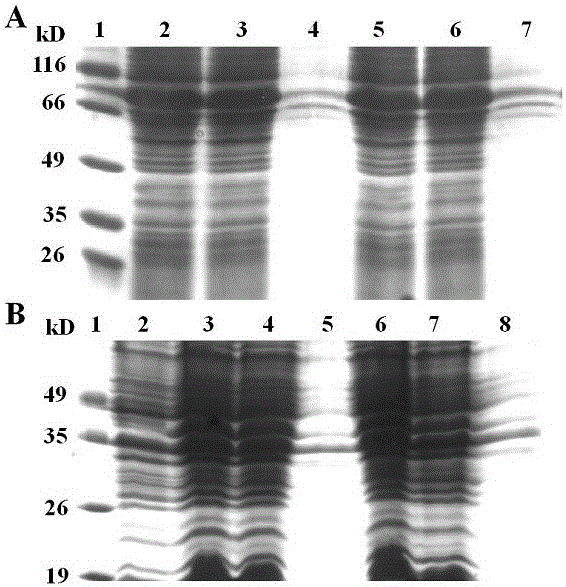
[0032] The four recombinant plasmids pET30a-hFGF21-HSA, pET30a-HSA-hFGF21, pET30a-hFGF21-3DHSA and pET30a-3DHSA-hFGF21 containing the correct sequence were respectively transformed into the expression strain Rosseta (DE3) (Beijing Quanshijin Biotechnology Co., Ltd. , catalog number: CD801). Transformed single colonies were inoculated into 20 mL of LB medium containing Kan (50 µg / mL), cultured at 37 °C for 8 h, and inoculated into another 20 mL of LB medium containing Kan (50 µg / mL) at a volume ratio of 1:100 Medium, cultured at 37°C, when A 600 At about 0.35, add IPTG to a final concentration of 0.25mmol / L for induction, and the induction temperature is 30°C. Harvest the cells after 5h, and use Binding buffer (20mmol / L Na 3 PO 4 , pH 7.0) to resuspend the bacteria, break the bacteria and centrifu...
Embodiment 3
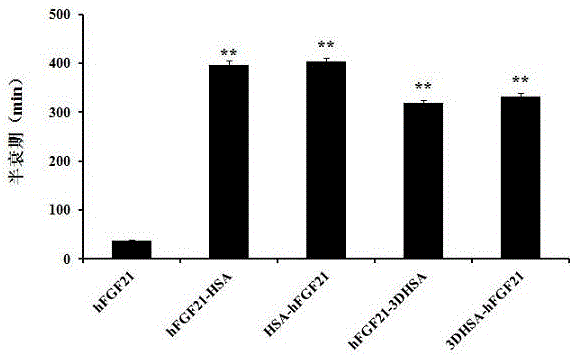
[0037] In vivo half-life detection of four proteins hFGF21-HSA, HSA-hFGF21, hFGF21-3DHSA and 3DHSA-hFGF21
[0038]Eighteen rabbits weighing about 2 kg were selected and randomly divided into 6 groups. Each group was subcutaneously injected with 6 kinds of proteins hFGF21, hFGF21-HSA, HSA-hFGF21, hFGF21-3DHSA and 3DHSA-hFGF21, the dose was 30nmol / kg, at 0h, 1h, 3h, 5h, 7h, 24h after administration, About 800 μL of blood was collected from the ear vein. Centrifuge at 12000r / m for 10min, take the supernatant and store it at -20°C for later use.
[0039] The in vivo half-life of six proteins was determined by ELISA indirect method: hFGF21, hFGF21-HSA, HSA-hFGF21, hFGF21-3DHSA and 3DHSA-hFGF21 proteins (2μg / mL, 0.2μg / mL, 200ng / mL, 20ng / mL and 2ng / mL) to establish the standard curve of protein concentration respectively, the diluted standard protein and serum were coated on the microtiter plate, and the content of the target protein in each serum was determined by ELISA indirect ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com