A dry powder inhaler with anti-lung cancer activity
A dry powder inhaler and dry powder inhalation technology, applied in the field of biomedicine, to achieve the effect of reducing drug dosage, high safety, and increasing concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
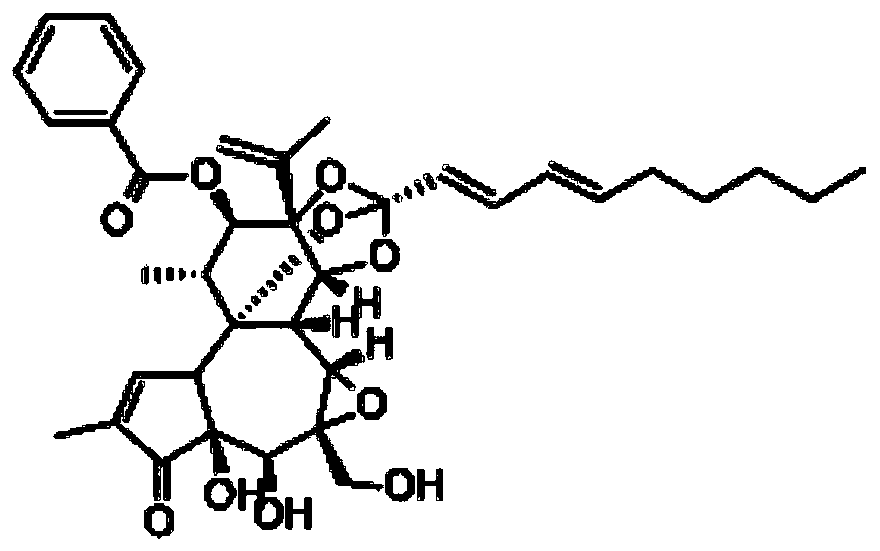
[0022] Embodiment 1: the establishment of the liquid phase condition of genkwa ester A and standard song
[0023] The test adopts Daphne A raw material drug, and the structural formula is as follows: figure 1 , to establish liquid phase analysis conditions, chromatographic column: Merck C18 column (4.6mm×250mm, 5μm), mobile phase: methanol-water (82:18), column temperature is 40°C, flow rate is 1.0mL / min, detection wavelength is 233nm, The injection volume is 20 μL. Carry out linear regression with peak area (A) as ordinate, concentration (C) as abscissa, obtain standard curve equation: A=19910C-12788(r 2 =1, the accuracy, precision and recovery rate are good, meeting the requirements of the analytical method), and the linear range is 2.5-80 μg / mL.
Embodiment 2
[0024] Embodiment 2: the preparation of genkwa ester A dry powder inhalation
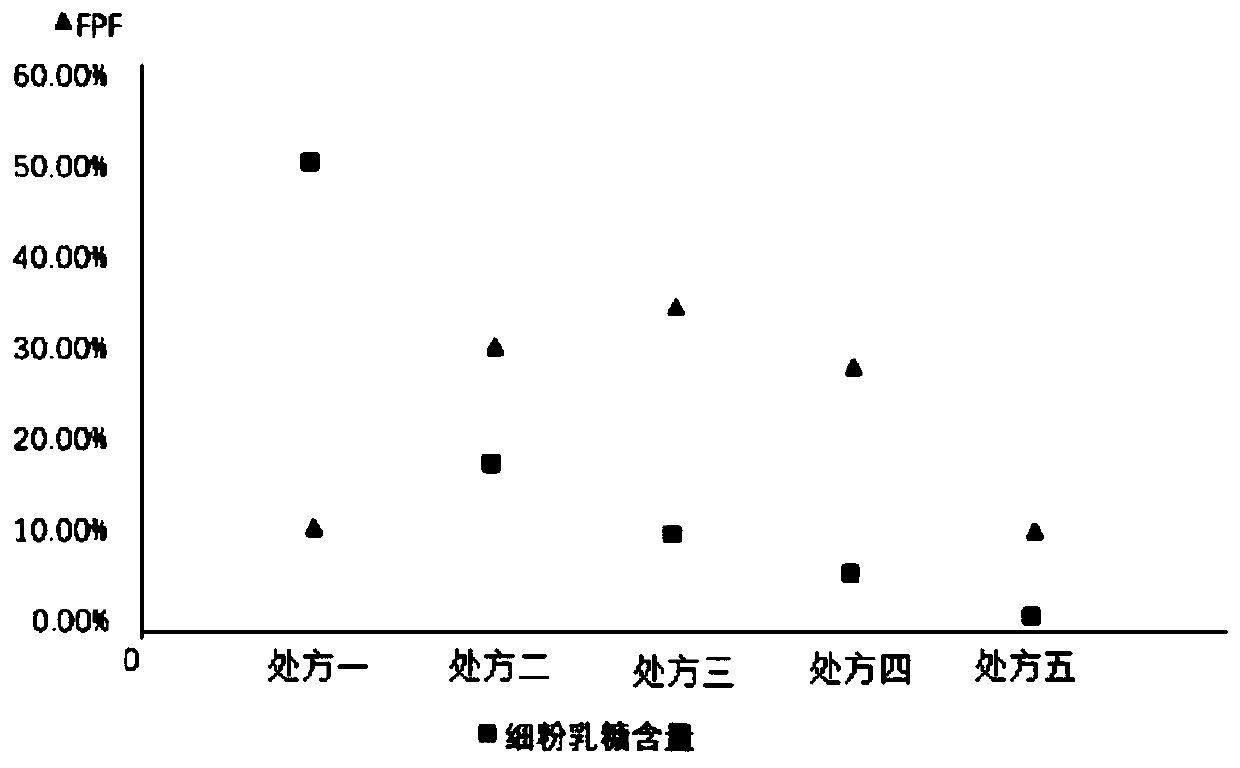
[0025] The lactose used in the prescription is coarse lactose (d>110 μ m) and fine powder lactose (d: 4-11 μ m), after changing the ratio of the two, it is made into a dry powder inhaler (DPI) to investigate the effect of lactose particle size on Daphne genkwa The effect of DPI on the performance of DPI administration in the lungs. The five prescriptions are shown in the table below. Weigh the rough lactose and fine powder lactose of the prescribed amount, and calculate the weight ratio of Daphne A and total lactose as 1:5. Weigh genkwa ester A and mix evenly.
[0026] Table 1 DPI prescription of genkwa ester A with different ratios of rough lactose and fine powder lactose
[0027]
Embodiment 3
[0028] Example 3: Experimental Method for Screening the Prescription of Genkwa Esperidin A Dry Powder Inhalation Preparation by Medicinal Impactor
[0029] Each prescription among the embodiment 2 is made into 20 drug-containing capsules respectively, and each capsule contains powder 50mg. Use the NGI device to simulate human lung administration at a flow rate of 60 L / min, rinse the collection trays and adapters with 50% methanol water, centrifuge at 12,000 r / min for 5 minutes, and take the supernatant and place it in a liquid phase vial. Determination of In Vitro Pulmonary Deposition of Daphne A DPI. The amount of drug deposition at all levels of the NGI device was calculated after sample injection. The results are shown in Table 2.
[0030] Table 2: The deposition amount of Daphne-methyl DPI in different formulations in the NGI device
[0031]
[0032]
[0033] Calculation results of in vitro deposition rate FPF
[0034] The in vitro deposition rate (FPF) is the mo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com