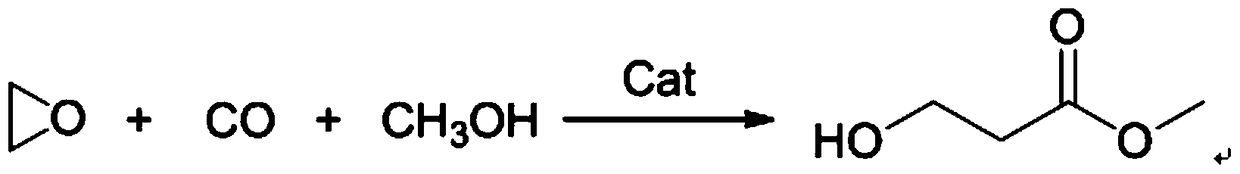
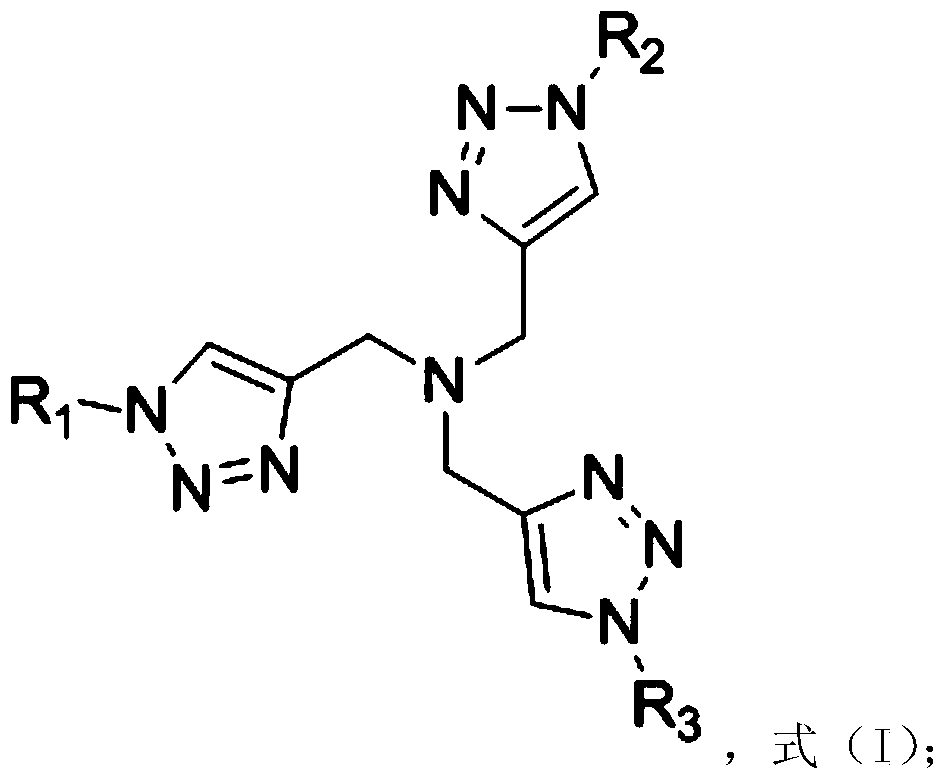
The synthetic method of 3-hydroxypropionate
A technology of hydroxypropionate and synthesis method, applied in chemical instruments and methods, carbon monoxide or formate reaction preparation, organic compound/hydride/coordination complex catalyst, etc., can solve the selection of 3-hydroxypropionate It can increase the activity, improve the stability, and improve the conversion rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
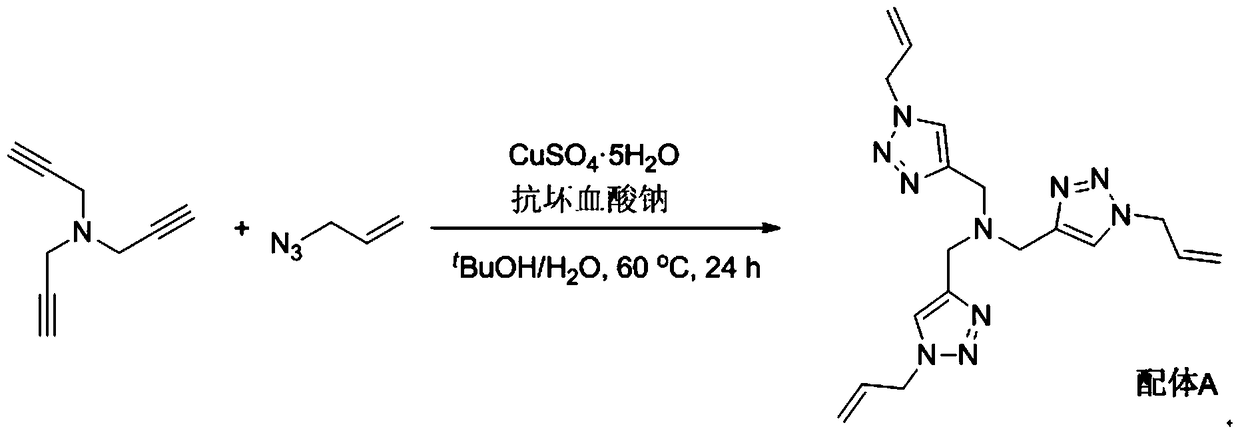
[0037] 1. Synthetic Ligand A
[0038]
[0039] Add 1312mg (10mmol) tripropargylamine, 2988mg (36mmol) allyl azide, 250mg (1mmol) copper sulfate hydrate (CuSO 4 ·5H 2 (0), 200mg (1mmol) sodium ascorbate, 40mL tert-butanol and 10mL water, the system was replaced with nitrogen three times, and reacted at 60°C for 24 hours under nitrogen atmosphere. After the reaction was completed, 100 mL of water was added, and the solution was extracted several times with dichloromethane. The organic phases were combined, dried, concentrated, and finally separated by column chromatography to obtain 1900 mg of light yellow solid which was Ligand A.
[0040] 2. Synthesis of methyl 3-hydroxypropionate
[0041] 342mg (1.0mmol) of Co 2 (CO) 8 Dissolve in 740 mmol of methanol, add 3.0 mmol of ligand A, and stir at room temperature for 2 hours. The catalyst solution was transferred to a 100 mL reactor, and the reactor was purged three times with nitrogen, and 50 mmol of ethylene oxide and carb...
Embodiment 2
[0044] 1. Synthetic Ligand B
[0045]
[0046] According to the experimental process of Example 1, with 1312mg (10mmol) tripropargyl amine, 3564mg (36mmol) allyl azide, 375mg (1.5mmol) copper sulfate hydrate (CuSO 4 ·5H 2 O), 300mg (1.5mmol) sodium ascorbate is raw material, synthesizes and obtains 1712mg brown solid and is ligand B.
[0047] 2. Synthesis of methyl 3-hydroxypropionate
[0048] 342mg (1.0mmol) of Co 2 (CO) 8Dissolve in 740 mmol of methanol, add 3.0 mmol of ligand B, and stir at room temperature for 2 hours. The catalyst solution was transferred to a 100 mL reactor, and the reactor was purged three times with nitrogen, and 50 mmol of ethylene oxide and carbon monoxide were added to make the system pressure 4.0 MPa, and the reaction was carried out at 50° C. for 4 hours. The kettle body was fully cooled to 0°C, and the pressure was slowly released to normal pressure. The reactor was purged three times with nitrogen, and the sampling analysis showed that t...
Embodiment 3
[0051] 1. Synthetic Ligand C
[0052]
[0053] According to the experimental process of Example 1, with 1312mg (10mmol) tripropargylamine, 4284mg (36mmol) phenyl azide, 250mg (1mmol) copper sulfate hydrate (CuSO 4 ·5H 2 O), 200mg (1mmol) sodium ascorbate is raw material, synthesizes and obtains 2196mg white solid and is ligand C.
[0054] 2. Synthesis of methyl 3-hydroxypropionate
[0055] 342mg (1.0mmol) of Co 2 (CO) 8 Dissolve in 740 mmol of methanol, add 3.0 mmol of ligand C, and stir at room temperature for 2 hours. The catalyst solution was transferred to a 100 mL reactor, and the reactor was purged three times with nitrogen, and 50 mmol of ethylene oxide and carbon monoxide were added to make the system pressure 4.0 MPa, and the reaction was carried out at 50° C. for 4 hours. The kettle body was fully cooled to 0°C, slowly released to normal pressure, purged the reactor three times with nitrogen, and sampled and analyzed, the results showed that the conversion ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com