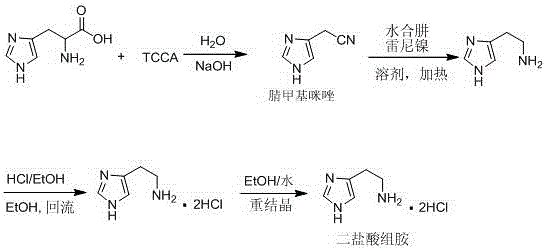
Synthetic method of histamine dichloride
A technology of histamine dihydrochloride and a synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of failing to meet medical standards, expensive raw materials, increase recovery costs, etc., and achieves shortening the process of acidification into salt, reducing production costs, and easy operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 293.0Kg of purified water and 6.00Kg of sodium hydroxide to a 1000L reactor, stir to dissolve completely, lower the temperature and stir to 20°C, add 23.00Kg of L-histidine, after the addition is complete, add 23.00Kg of TCCA in batches, about 1.0 After the addition was completed within 1 hour, the reaction was carried out with heat preservation and stirring for about 30 minutes. TLC monitoring was performed until the reaction of histidine was complete (developing solvent: ethyl acetate). After the reaction, add about 18.00kg of anhydrous sodium carbonate, adjust the pH value of the system to ≈8, shake off the filter, discard the filter cake, concentrate the filtrate under reduced pressure until solids are precipitated, and add 72.00Kg of tetrahydrofuran / acetic acid to the remaining concentrated solution Mixed solution of ethyl ester (tetrahydrofuran: ethyl acetate = 1: 1), stirred for 30 minutes, allowed to stand to separate layers, and the aqueous phase was extract...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com