Preparation method of phosphorus and nitrogen contained acrylate and copolymer core-shell particles of phosphorus and nitrogen contained acrylate
A technology of acrylate and butyl acrylate, which is applied in the preparation of phosphorus-nitrogen-containing acrylate and its copolymer core-shell particles, in the field of polymer chemistry and polymers, can solve the problems of high synthesis cost and unsuitability for industrialization, and achieve composition adjustable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
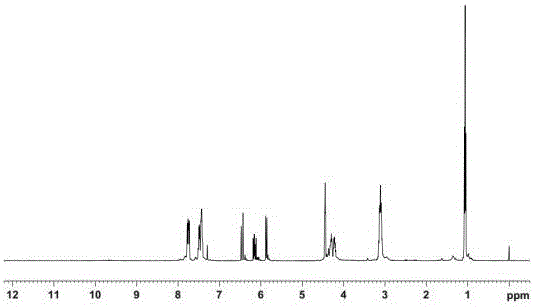
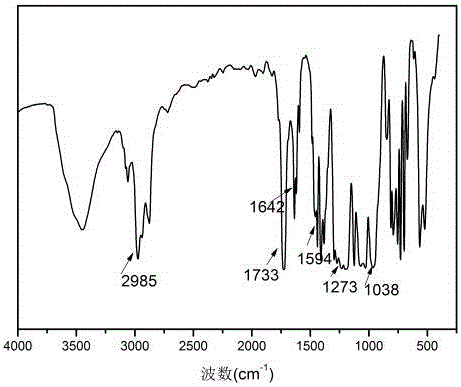
[0023] Add 19.5g (0.1mol) of phenylphosphoryl dichloride (PDCP), 25.3g (0.25mol) of triethylamine (TEA) and 80mL of solvent tetrahydrofuran (THF) into a three-necked flask equipped with a stirrer and cool down to -5 -0°C, add 11.6g (0.1mol) of hydroxyethyl acrylate (HEA) dropwise, react at 0-5°C for 3 hours, add 7.3g (0.1mol) of diethylamine (DEA) dropwise, After reacting at ℃ for 2 hours, raise the temperature to room temperature and react for 4 hours. The reaction mixture is filtered to remove triethylamine hydrochloride, and the obtained filtrate removes the solvent and unreacted triethylamine to obtain a crude product; the product is mobile with ethyl acetate. The phase was purified by neutral silica gel column chromatography, and the light yellow viscous liquid was obtained after removing ethyl acetate, which was phosphorus-nitrogen-containing acrylate.
Embodiment 2
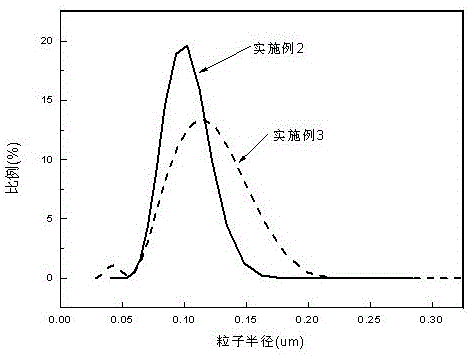
[0025] First add 80ml of distilled water to a 250ml four-neck flask equipped with an electric stirrer, a thermometer, and a reflux condenser, then turn on the stirring device, add 0.4gNaHCO3, 2.5gSDS and 1.5gOP-10 to make it stir slowly. After it dissolves, add 0.1g KPS and start to heat up. After the temperature rises to about 60°C, add 20g of inner core monomer (a mixture of 15g of phosphorus nitrogen-containing acrylate and 10g of butyl acrylate), raise the temperature to 70-75°C, initiate polymerization, and then add dropwise The remaining 36g of inner core monomer (a mixture of 10g of phosphorus nitrogen-containing acrylate and 26g of butyl acrylate) was reacted for 2 hours, and 0.1g of KPS was added, and 21g of methyl methacrylate, a shell monomer, was added dropwise, and the reaction was continued for 3 hours. After the emulsion is demulsified, copolymer core-shell particles with particle diameters ranging from 0.05 to 0.16 microns are obtained.
Embodiment 3
[0027] First add 80ml of distilled water to a 250ml four-neck flask equipped with an electric stirrer, a thermometer, and a reflux condenser, then turn on the stirring device, add 0.4gNaHCO3, 2.5gSDS and 1.5gOP-10 to make it stir slowly. After it dissolves, add 0.1g KPS and start to heat up. After the temperature rises to about 60°C, add 20g of inner core monomer (a mixture of 16g of phosphorus-nitrogen acrylate and 4g of butyl acrylate), raise the temperature to 70-75°C, initiate polymerization, and then add dropwise The remaining 30g of inner core monomer (a mixture of 24g of phosphorus-nitrogen acrylate and 6g of butyl acrylate) was reacted for 2 hours, and 0.1g of KPS was added, and 25g of methyl methacrylate, a shell monomer, was added dropwise, and the reaction was continued for 3 hours. After the emulsion is demulsified, copolymer core-shell particles with a particle size of 0.05-0.23 microns are obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com