Method for manufacturing metal powder
A metal powder and metal technology, applied in metal processing equipment, transportation and packaging, non-polymer adhesive additives, etc., can solve problems such as low thermal conductivity and electrical conductivity, and fatigue failure of soft solder
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-A
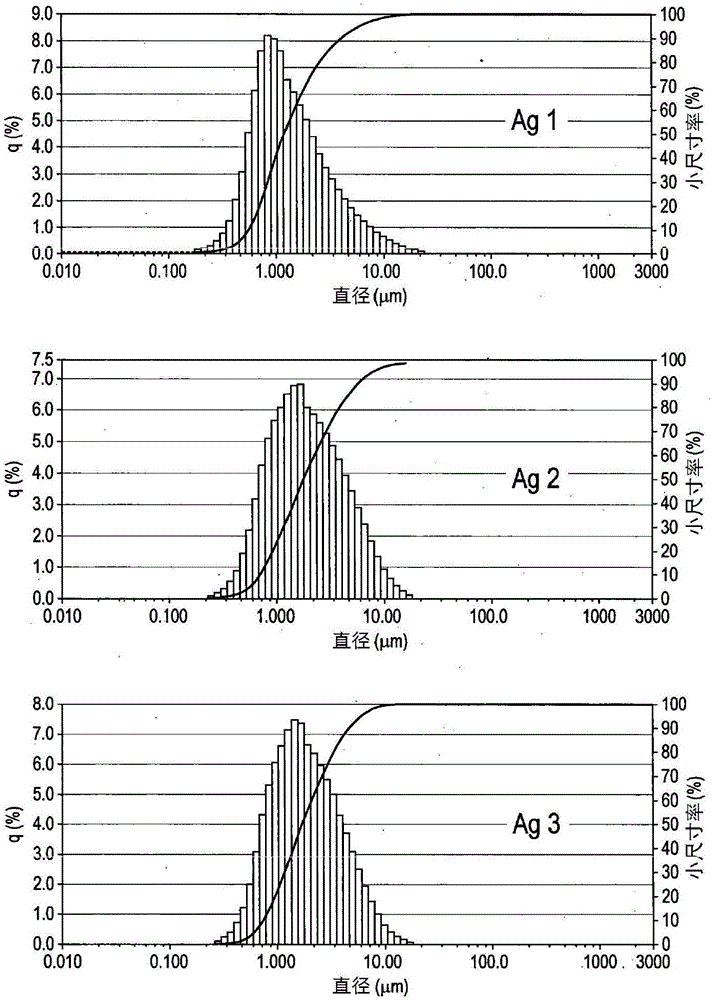
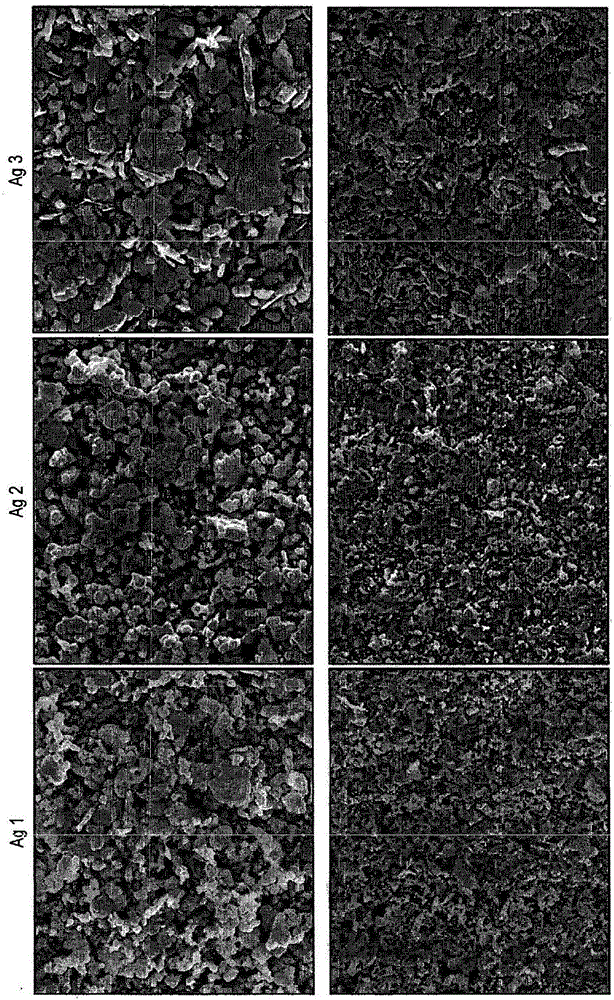
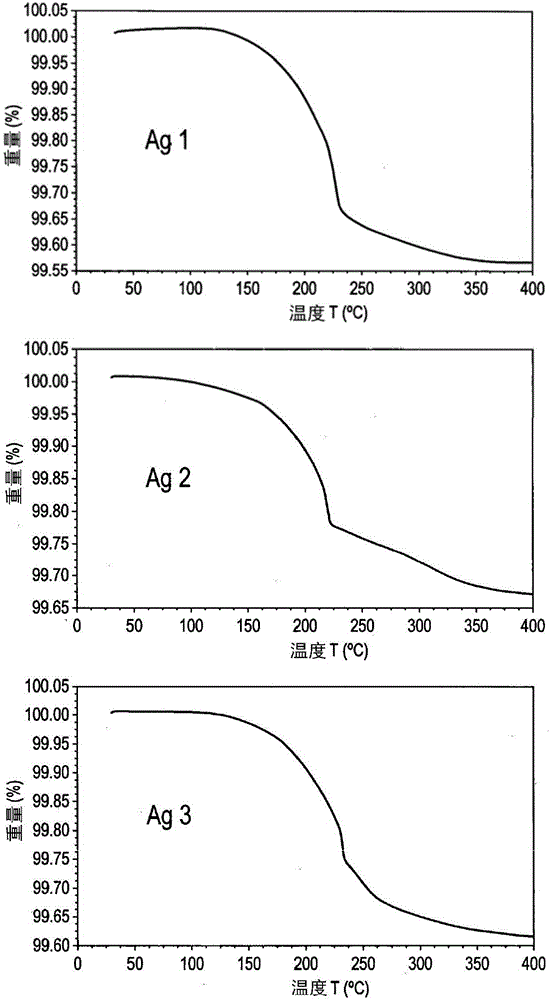
[0168] Example 1 - Ag microparticle type - 1
[0169] In the chemical process, 420 g of silver nitrate was dissolved in 2100 g of deionized water by stirring. To this was added 420 g of DMF and stirring continued. To this solution were added simultaneously two separate solutions, (1) a mixture of 325 g triethylamine and 460 g formaldehyde, and (2) a solution of 30 g sodium hydroxide in 200 g deionized water. A freshly prepared sodium oleate solution was then added immediately (6.3 g oleic acid was added to a solution of 1.3 g sodium hydroxide in 200 g water). The mixture was stirred for 1 hour, then the powder was filtered, washing with water and acetone until the pH of the filtrate was neutral. The powder was then dried in an oven at 70°C for 8 hours.
[0170] This was followed by a mechanical process in which 200 g of the dried powder was stirred in a solution of 250 g toluene and 4 g oleic acid for 30 min and then mechanically treated using zirconia beads with a size o...
Embodiment 2 and 3-A
[0171] Example 2 and 3 - Ag Microparticle Type - 2 and 3
[0172] In the chemical process, 420 g of silver nitrate was dissolved in 1500 g of deionized water by stirring. To this was added a solution of 89 g of sodium hydroxide in 400 g of deionized water, resulting in a brown heterogeneous solution. A solution of 126 g of hydrazine hydrate in 1890 g of deionized water was added to the reaction mixture, followed by a freshly prepared solution of sodium oleate (6.3 g of oleic acid was added to a solution of 1.3 g of sodium hydroxide in 210 g of water). The mixture was stirred for 1 hour, after which the powder was filtered, washed with water and acetone until the pH of the filtrate was neutral. The powder was then dried in an oven at 70°C for 8 hours.
[0173] This was followed by a mechanical process in which 200 g of the dried powder was stirred in a solution of 200 g of toluene and 4 g of oleic acid for 30 min and then mechanically treated (a) for 4.5 h using vertical ag...
Embodiment 4
[0174] Example 4 - Preparation of multifaceted copper microparticle type Cu2
[0175] 1140 g of copper(II) nitrate trihydrate were dissolved in 1550 g of deionized water containing 6.9 g of surfactant (DAXAD) by stirring for 30 minutes. The reaction mixture was kept on a hot plate with an electronic thermometer. 1380 mL of 30% ammonia solution was added to the above solution until the pH became 8. The solution was stirred for 10 minutes. The temperature was set at 70°C. After reaching the desired temperature, 1970 mL of 60% hydrazine hydrate was added at a rate of 30 mL per minute and stirring was continued for 30 minutes. To reduce effervescence, add a minimum amount of ethanol at regular intervals. The temperature was set at 85°C. After reaching temperature, the solution was stirred for 2.5 hours. The powder was allowed to settle and collected by decanting the supernatant. It was washed with water and acetone and dried in a forming gas (90-95% nitrogen: 5-10% hydrog...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tap density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com