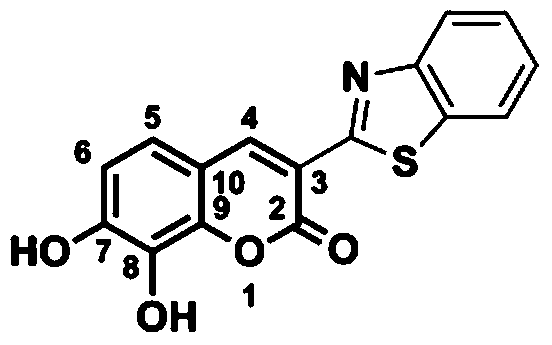
Specific fluorescent probe for catechol-o-methyltransferase and its application
A technology of methyltransferase and fluorescent probe, which is applied in the field of biomedicine, can solve the problems of poor compound stability, complicated sample pretreatment, and inconvenient quantitative detection, etc., and achieve high sensitivity, good fluorescence emission spectrum characteristics, and simple synthesis process line effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
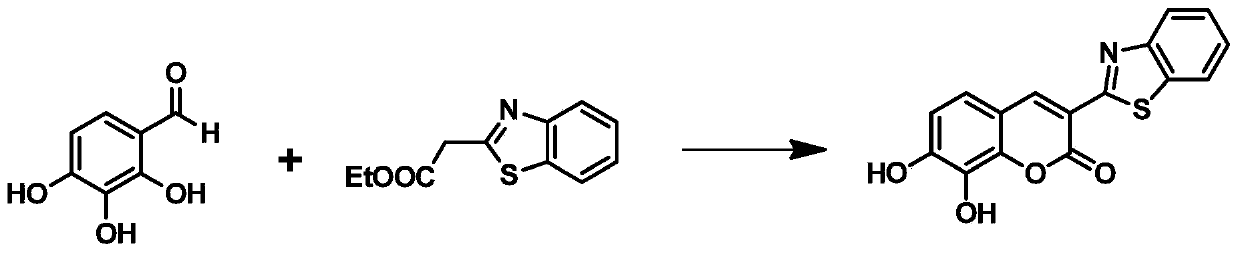
[0045] .3-Benzothiazole-7,8-dihydroxycoumarin synthesis
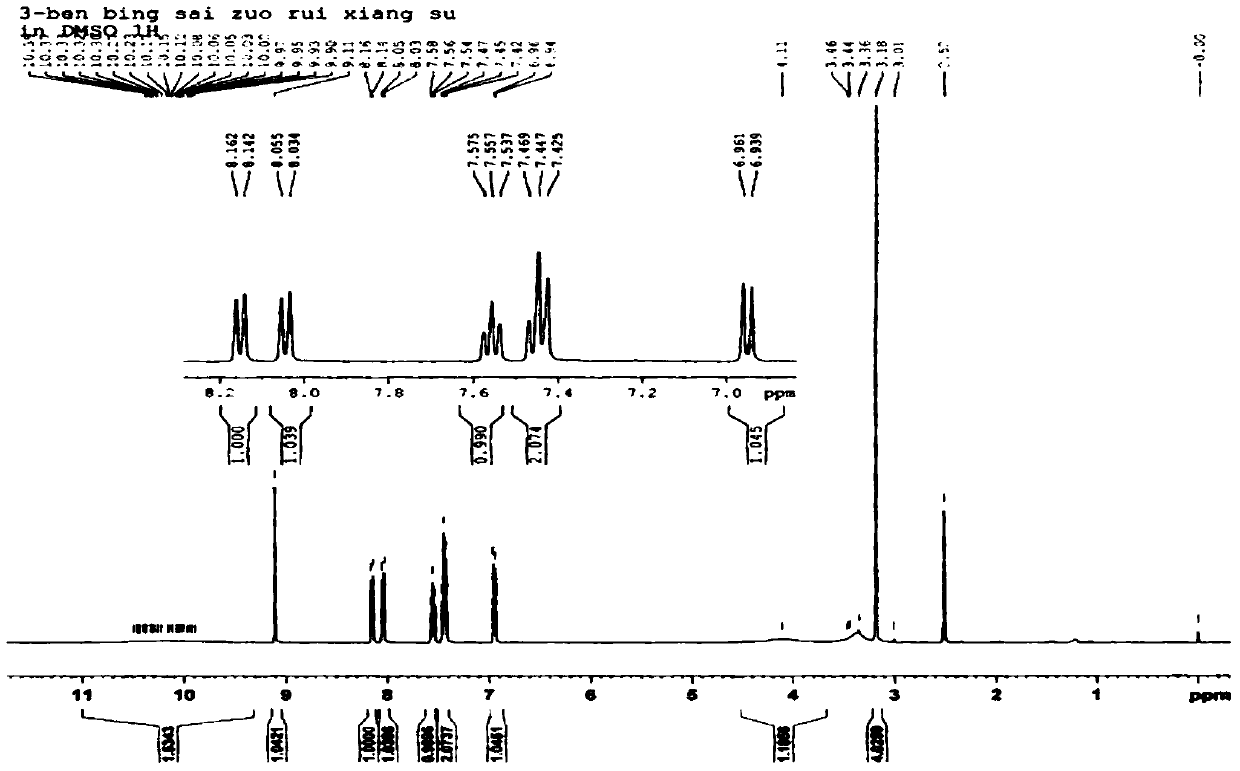
[0046] The synthetic route of 3-benzothiazole-7,8-dihydroxycoumarin is as follows figure 2 As indicated, weigh 1.0 g of 2,3,4-trihydroxybenzaldehyde and dissolve it in 25 mL of methanol, add 1.5 mL of 2-(2-benzothiazole) ethyl acetate and 0.1 mL of piperidine, and stir at room temperature for 20 minutes. Heating to 65° C., reacting for 4 h, and TLC detected the end of the reaction. After the reaction solution was cooled, 10 mL of water was added, filtered, and the filter cake was collected, which was the crude product. The crude product was added to 20 mL of acetonitrile and refluxed for 1 h, filtered after cooling, and dried in vacuo to obtain 1.8 g of the product 3-benzothiazole-7,8-dihydroxycoumarin as a brownish yellow solid with a yield of 88%. 1 H NMR spectrum and 13 C NMR spectrum as image 3 and Figure 4 shown;
[0047] 1 H NMR (400MHz, DMSO-d 6 )δ: 6.95(d, J=4.4Hz, 1H), 7.45(m, 2H), 7.56(t, J=8.0Hz, 1...
Embodiment 2
[0051] Determination of the lower limit of detection of COMT in vitro
[0052] The experiment was carried out on a microplate reader using a 96-well plate, 3-benzothiazole-7,8-dihydroxycoumarin 5μM, S-adenosylmethionine 200μM, dithiothreitol 2mM, MgCl 2 5mM, COMT single enzyme 20ng / ml~200μg / ml, pH 7.4 Tris-HCl buffer 50mM, the total volume is 200μL, incubated at 37℃ for 5min and analyzed by microplate reader, the average value of each group is the same as that without COMT Compared with the control group, it was shown that the COMT of 40ng / ml was statistically significant (P Figure 8 ).
Embodiment 3
[0054] COMT time standard curve determination
[0055] The experiment was carried out on a microplate reader using a 96-well plate, 3-benzothiazole-7,8-dihydroxycoumarin 5μM, S-adenosylmethionine 200μM, dithiothreitol 2mM, MgCl 2 5mM, COMT single enzyme 20ng / ml~200ng / ml, pH 7.4 Tris-HCl buffer 50mM, total volume 200μL, incubate at 37℃ for 5min, analyze with a microplate reader every 1 minute, the fluorescence intensity of the product and the incubation time Make a standard curve, the R of each standard curve 2 >0.99 (see Figure 9 ), indicating that the standard curve has a wide linear range and can accurately quantify the content of COMT.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com