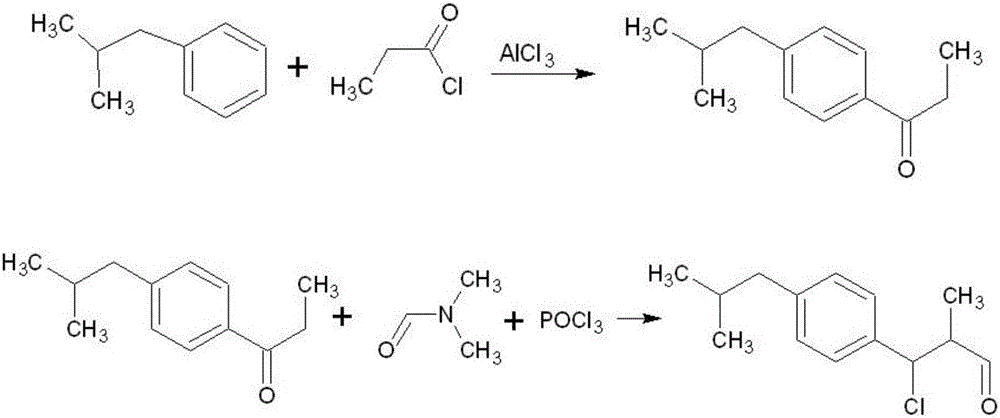
Synthesis method of p-isobutyl-beta-chloro-alpha-methyl allyl benzene aldehyde
A technology of methacrolein and a synthesis method, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, and preparation of carbonyl compounds by condensation, etc., can solve the problems of high production cost, environmental pollution, large amount of waste water, etc. Control, high yield, and easy industrial-scale production effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] a) First add 140g of dichloroethane into a 500ml four-necked distillation flask, then put in 60g of aluminum trichloride, turn on the magnetic stirrer and constant temperature water bath, and cool down to -5±5°C;
[0045] b) Add 40 g of propionyl chloride dropwise to the distillation flask through the drop pump, control the drop time for 4 hours, control the reaction temperature to -5±5°C, and continue stirring for 0.5 h after the dropwise addition;
[0046] c) Continue to keep the temperature at -5±5°C, add 55g of isobutylbenzene dropwise through the dropping pump, and control the dropping time at 2h. After the dropping, raise the temperature to 20-30°C, and continue stirring for 0.5h;
[0047] d) Sampling detection, when the content of isobutylbenzene is ≤0.5%, reduce the temperature of the reaction system to -15~-10°C, increase the stirring rate, slowly add 175g of clear water for extraction, terminate the reaction, during the extraction process, control Reactor temper...
Embodiment 2
[0059] a) First add 700g of dichloroethane into a 2500ml four-necked distillation flask, then put in 300g of aluminum trichloride, turn on the magnetic stirrer and constant temperature water bath, and cool down to -5±5°C;
[0060] b) Add 200 g of propionyl chloride dropwise to the distillation flask through the drop pump, control the drop time for 6 hours, control the reaction temperature at -5±5°C, and continue stirring for 1 hour after the dropwise addition;
[0061] c) Continue to keep the temperature at -5±5°C, add 275g of isobutylbenzene dropwise through the dropping pump, control the dropping time at 2h, raise the temperature to 20-30°C after the dropping, and continue stirring for 1h;
[0062] d) Sampling and detection, when the content of isobutylbenzene is ≤0.5%, reduce the temperature of the reaction system to -15~-10°C, increase the stirring rate, slowly add 900g of clear water for extraction, terminate the reaction, and control Reactor temperature <40°C;
[0063] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com