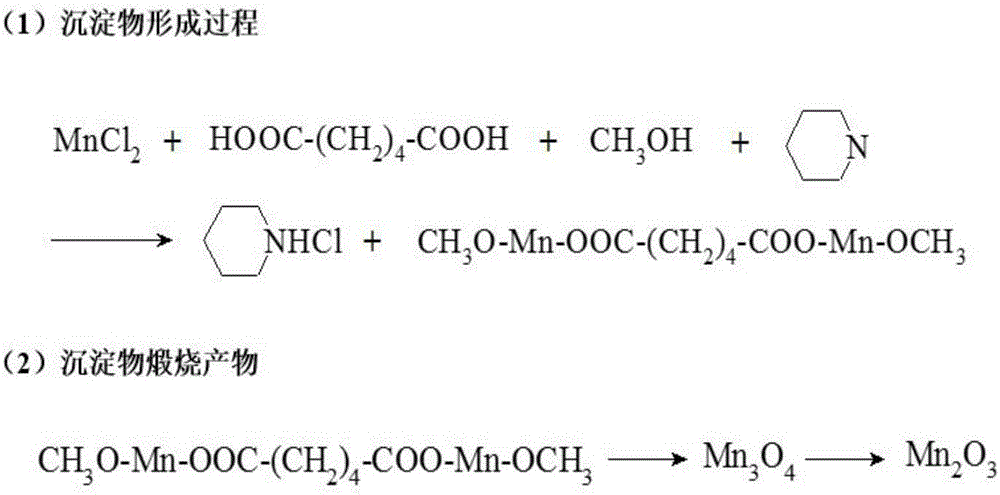Hydroxyl-containing manganese-series catalyst used for low-temperature flue gas denitration and preparation method thereof
A hydroxy manganese-based, low-temperature flue gas technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve problems such as poor selectivity and poor sulfur resistance , to achieve the effect of convenient operation, simple process and good anti-sulfur poisoning performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (a) Accurately weigh 11.874g of manganese dichloride tetrahydrate and 4.380g of adipic acid, dissolve them in 120mL methanol solution together, stir well, and prepare solution A;
[0028] (b) Accurately measure 15mL of piperidine solution, add dropwise to 105mL of methanol solution, stir evenly, and prepare solution B;
[0029] (c) rapidly stirring the solution A in the step (a), during which, the solution B of the step (b) is slowly added dropwise to the rapidly stirring solution (a), until the reaction is complete, and a precipitate C is formed;
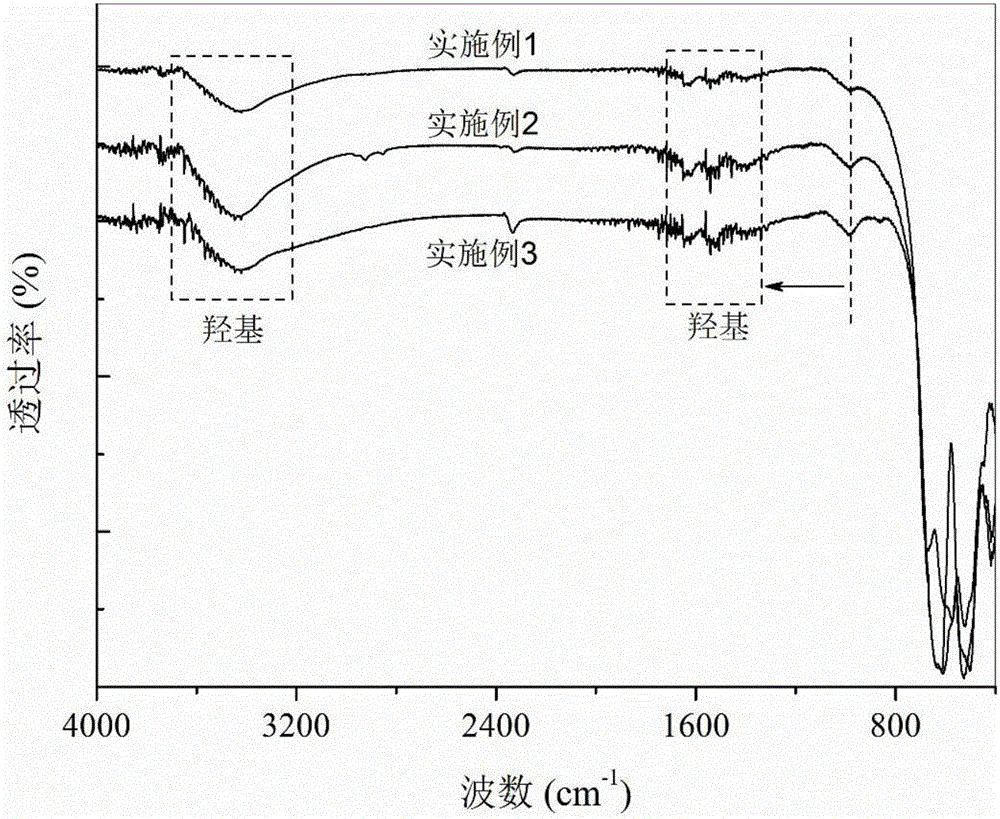
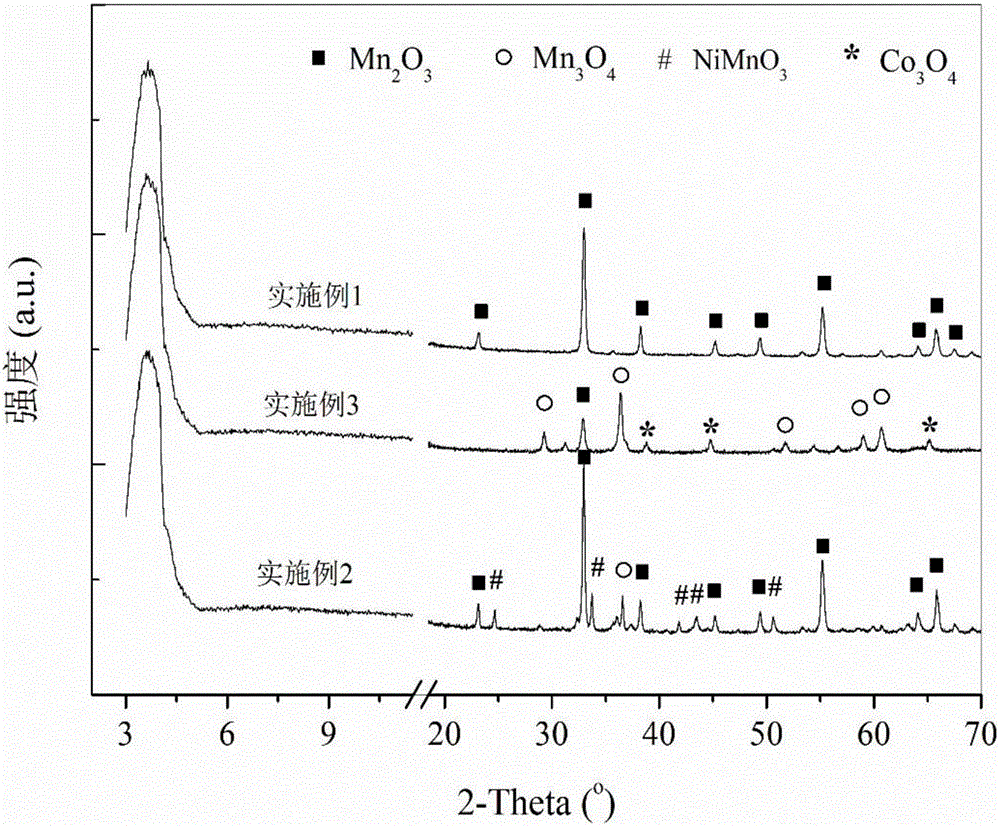
[0030] (d) Seal and age the mixed solution containing precipitate C in step (c) at room temperature for 12 hours, filter the precipitate, wash with excess methanol, and dry in vacuum at 65°C for 12 hours; FTIR spectrum of the dried sample Figure and XRD spectrum as attached figure 2 And attached image 3 ;
[0031] (e) Place the dried sample in step (d) in a muffle furnace, set the calcination temperature to 550°C, and s...
Embodiment 2
[0036](a) Accurately weigh 11.874g of manganese dichloride tetrahydrate, 4.754g nickel dichloride hexahydrate and 4.380g of adipic acid, dissolve in 120mL methanol solution together, stir well, and be mixed with solution A;
[0037] (b) Accurately measure 15mL of piperidine solution, add dropwise to 105mL of methanol solution, stir evenly, and prepare solution B;
[0038] (c) rapidly stirring the solution A in the step (a), during which, the solution B of the step (b) is slowly added dropwise to the rapidly stirring solution (a), until the reaction is complete, and a precipitate C is formed;
[0039] (d) Seal and age the mixed solution containing precipitate C in step (c) at room temperature for 12 hours, filter to obtain the precipitate, wash with excess methanol, and vacuum-dry at 65°C for 12 hours; FTIR spectrum of the dried sample The picture is attached figure 2 ;
[0040] (e) Place the dried sample in step (d) in a muffle furnace, set the calcination temperature to 55...
Embodiment 3
[0045] (a) Accurately weigh 11.874g of manganese dichloride tetrahydrate, 4.758g of cobalt dichloride hexahydrate and 4.380g of adipic acid, dissolve them in 120mL methanol solution together, stir well, and prepare solution A;
[0046] (b) Accurately measure 15mL of piperidine solution, add dropwise to 105mL of methanol solution, stir evenly, and prepare solution B;
[0047] (c) rapidly stirring the solution A in the step (a), during which, the solution B of the step (b) is slowly added dropwise to the rapidly stirring solution (a), until the reaction is complete, and a precipitate C is formed;
[0048] (d) Seal and age the mixed solution containing precipitate C in step (c) at room temperature for 12 hours, filter to obtain the precipitate, wash with excess methanol, and vacuum-dry at 65°C for 12 hours; FTIR spectrum of the dried sample The picture is attached figure 2 ;
[0049] (e) Place the dried sample in step (d) in a muffle furnace, set the calcination temperature to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com