3-bromobutyl-5,5-dimethylhydantoin and preparation method thereof
A technology of dimethyl hydantoin and bromobutyl, applied in the field of 3-bromobutyl-5,5-dimethylbromobutyl hydantoin and its preparation, can solve the problems of long time-consuming and complicated operation, and achieve sterilization The effect is good, the synthesis process is optimized, and the sterilization speed is fast.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
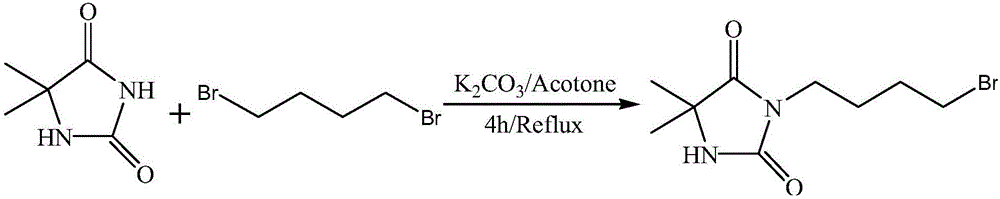
[0032] A kind of preparation method of the present invention prepares 3-bromobutyl-5,5-dimethyl hydantoin, specifically comprises the following steps:
[0033] 1) 5,5-dimethylhydantoin is added to the acetone solution containing acid-binding agent, and the mass ratio of 5,5-dimethylhydantoin to acid-binding agent is: 1:(3- 5), stirring and refluxing to fully react;
[0034] Acid binding agent is anhydrous K 2 CO 3 , anhydrous Na 2 CO 3 , NaOH, KOH or triethylamine, the stirring and reflux time is 20-60min.
[0035] 2) Add 1,4-dibromobutane, control the water temperature and continue to reflux until fully reacted to make a solution, the molar ratio of 5,5-dimethyl hydantoin to 1,4-dibromobutane is 1:(1.5 -3); the reflux time is 3-7h, and the water temperature is 58°C-60°C;
[0036] 3) Suction filter the solution obtained in step 2) to remove inorganic salts, concentrate, add ethyl acetate and water for extraction and liquid separation, take the supernatant, remove ethyl a...
Embodiment 1
[0038] Dissolve 5,5-dimethylhydantoin (1g, 7.788mmol) in 70mL of acetone, stir to dissolve, add anhydrous K 2 CO 3 (4g), heated to reflux for 30min, then added 1,4-dibromobutane (3.37g, 15.6mmol) to the mixture, and continued to reflux for 5h to obtain a white suspension. The solvent was removed by rotary evaporation to obtain a clear viscous liquid.
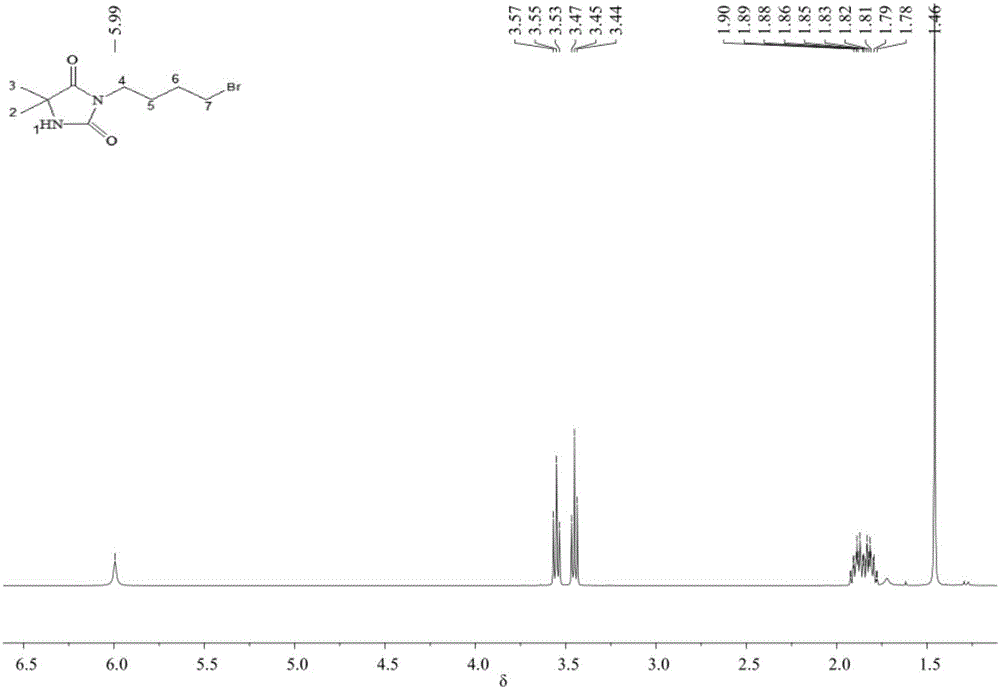
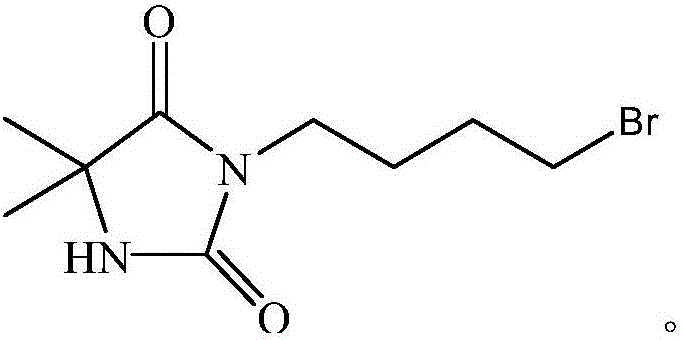
[0039] Water and ethyl acetate were added thereto for extraction and liquid separation, and the supernatant was taken; after ethyl acetate was removed, column chromatography was performed using ethyl acetate / petroleum ether as the eluent. Collect the obtained liquid containing the product, and remove the eluent to obtain a white solid that is 3-bromobutyl-5,5-dimethylhydantoin, the structure of which is as follows: 1 H NMR (400MHz, CDCl 3 )δ5.94(s, 1H), 3.55(t, J=6.8Hz, 3H), 3.45(t, J=6.4Hz, 3H), 1.94–1.79(m, 6H), 1.70(s, 1H), 1.46(s, 8H).
Embodiment 2
[0041] Dissolve 5,5-dimethylhydantoin (1g, 7.788mmol) in 70mL of acetone, stir to dissolve, add anhydrous Na 2 CO 3 (4g), heated to reflux for 30min, then added 1,4-dibromobutane (3.37, 15.6mmol) to the mixture, and continued to reflux for 5h to obtain a white suspension. The solvent was removed by rotary evaporation to obtain a clear viscous liquid.
[0042] Water and ethyl acetate were added therein for extraction and liquid separation, and the supernatant was taken; after ethyl acetate was removed, column chromatography was performed with ethyl acetate / petroleum ether as eluent. The resulting liquid containing the product was collected, and the white solid obtained after removing the eluent was 3-bromobutyl-5,5-dimethylhydantoin, the structure of which was as follows: 1 H NMR (400MHz, CDCl 3 )δ5.94(s, 1H), 3.55(t, J=6.8Hz, 3H), 3.45(t, J=6.4Hz, 3H), 1.94–1.79(m, 6H), 1.70(s, 1H), 1.46(s, 8H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com