Injectable hyaluronic acid/polyethylene glycol hydrogel and its preparation method and application
A technology of polyethylene glycol and hyaluronic acid, which can be used in pharmaceutical formulations, prostheses, drug delivery, etc. It can solve the problems of poor plasticity and durability, and achieve good mechanical properties, low cytotoxicity, and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Synthesis of azide-modified polyethylene glycol
[0040] (1) Synthesis of terminal diepoxy PEG
[0041] Terminated Diepoxide PEG
[0042] 20.2g PEG (M n =6000) was placed in a 500mL flask, vacuum-dried with an oil pump for 3h under the condition of heating in an oil bath at 120°C, and then added 200mL of anhydrous tetrahydrofuran, dissolved until clear and transparent, and then added 1.35g of sodium hydride. After stirring overnight at room temperature, 5.3 mL of epichlorohydrin was added, reacted at room temperature for 20 h, and then filtered. The filtrate was concentrated and precipitated in ether, and the resulting solid was vacuum-dried to obtain 19.0 g of white solid-terminated diepoxy PEG with a yield of 94%. 1 H NMR (400MHz, CDCl 3 ,TMS):δ3.57-3.74(m,CH 2 of PEG chain), 3.39(m,2H), 3.1(m,2H), 2.72(m,2H), 2.54(m,2H); 13 CNMR (100MHz, CDCl 3 , TMS), δ70.5, 72.8, 50.4, 44.2.
[0043] (2) Synthesis of terminal tetrahydroxy PEG
[0044] Tetrahydr...
Embodiment 2
[0052] Example 2: Synthesis of Cyclooctyne Modified Hyaluronic Acid
[0053] (1) Synthesis of N-(2-((Z)-2-bromocyclooct-2-enyloxy)ethyl)trifluoroacetamide
[0054] N-(2-((Z)-2-bromocyclooct-2-enyloxy)ethyl)trifluoroacetamide
[0055] First, 4.31g of silver trifluoromethanesulfonate was placed in a 50mL three-necked flask, which was evacuated and filled with argon three times; under the protection of argon, 8mL of toluene was injected into the flask, and then 10.81g of N-(2-hydroxyethyl)trifluoro A mixed solution of acetamide and 1.50 g of 8,8-dibromobicyclo[5.1.0]octane was added dropwise into the flask while stirring. After one day of reaction in the dark, 50 mL of saturated brine was added to quench the reaction, suction filtered, and the filtrate was extracted with dichloromethane (50 mL×3), the organic phases were combined and dried over anhydrous sodium sulfate, filtered, and the solvent was spin-dried to obtain 5.14 g of crude product. The crude product was subjected...
Embodiment 3
[0062] Embodiment 3: SPAAC reaction prepares hydrogel
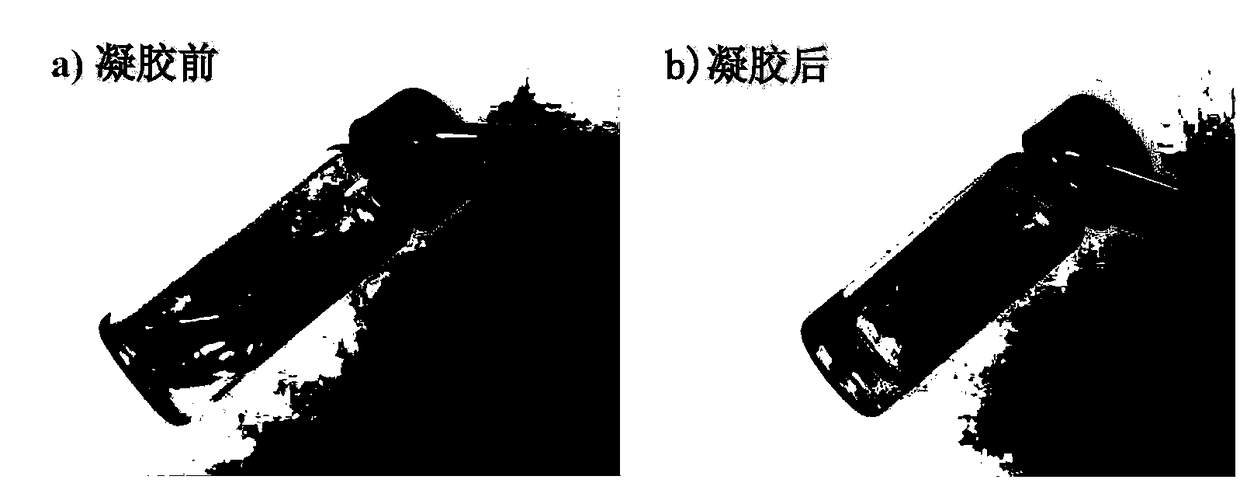
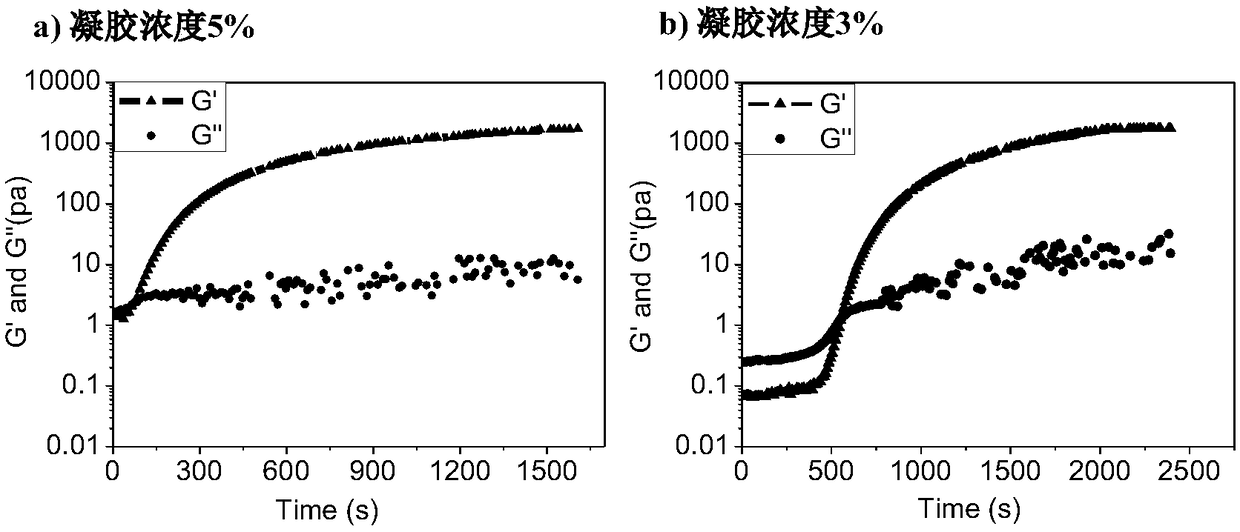
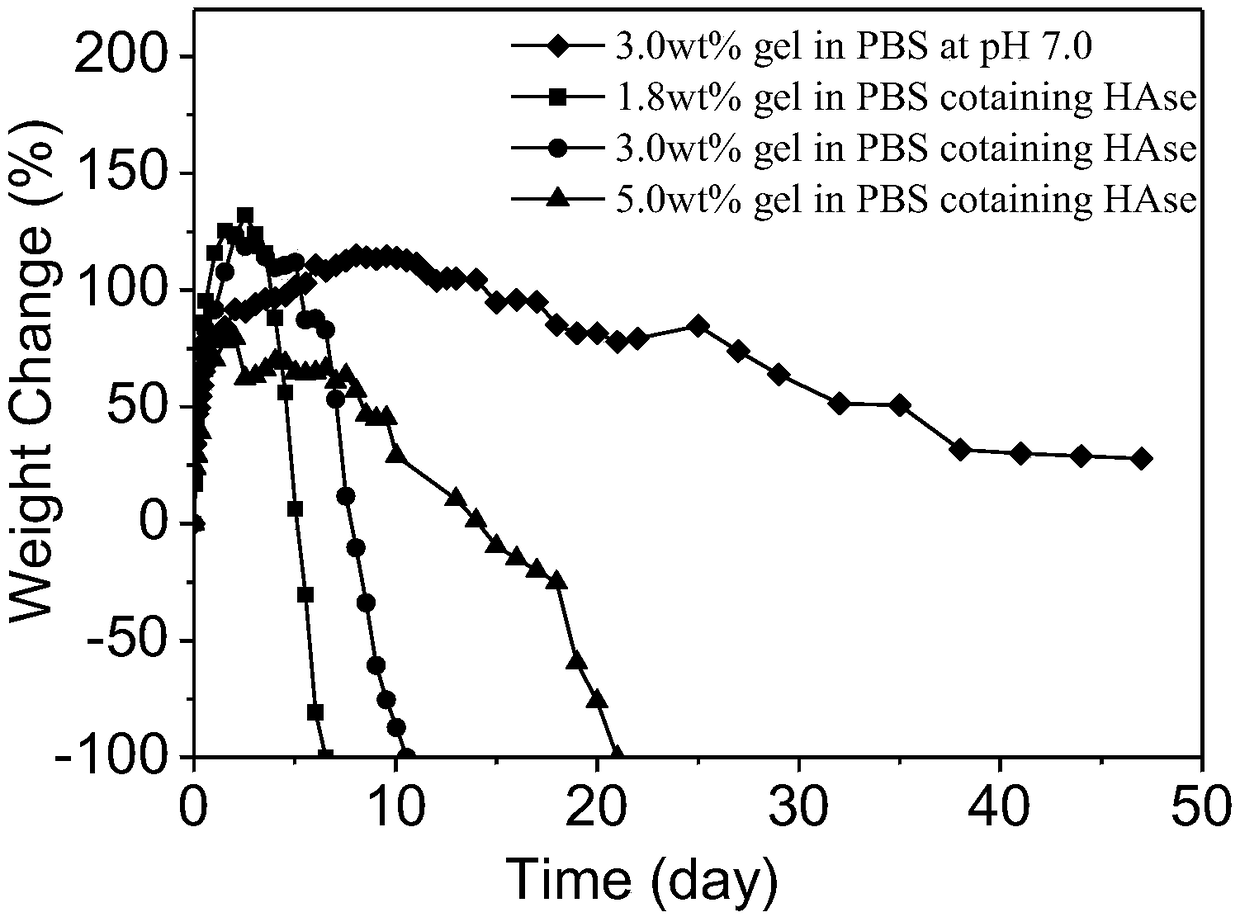
[0063] The azide-modified polyethylene glycol prepared in Example 1 and the cyclooctyne-modified hyaluronic acid prepared in Example 2 were respectively dissolved in PBS buffer solution with pH = 7.4, and the concentration range of cyclooctine was 1wt%-10wt%. Alkyne-modified hyaluronic acid solution and azide-modified polyethylene glycol solution whose concentration was changed according to the molar amount of alkyne functional group. Mix the cyclooctyne-modified hyaluronic acid solution and the azide-modified polyethylene glycol solution, vortex for half a minute, put it in a constant temperature water bath at 37°C, and let it stand for 3-50 minutes to obtain different concentrations and molar ratios of alkynyl / alkyne groups. Azide = 1:0.5, 1:1, 1:2, 1:3 hydrogels. figure 1 It is a comparison picture of the hydrogel before and after.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com