Preparation and application of antimicrobial peptide fusion cytokine campils co-expression biological preparation
A technology of biological materials and immune cells, applied in the direction of fusion polypeptide, antibody mimic/scaffold, cells modified by introducing foreign genetic material, etc., can solve problems such as decreased immune resistance, threats to human health, and weakened health levels , to inhibit the growth of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Embodiment 1, antimicrobial peptide (CAMPILs) has bacteriostasis
[0118] The present invention provides an antimicrobial peptide named CAMPILs, its amino acid sequence is shown in sequence 1 in the sequence listing, and the nucleotide sequence encoding CAMPILs is shown in sequence 2.
[0119] 1. Preparation of recombinant yeast SMDpG-P
[0120] 1. Preparation of recombinant bacteria
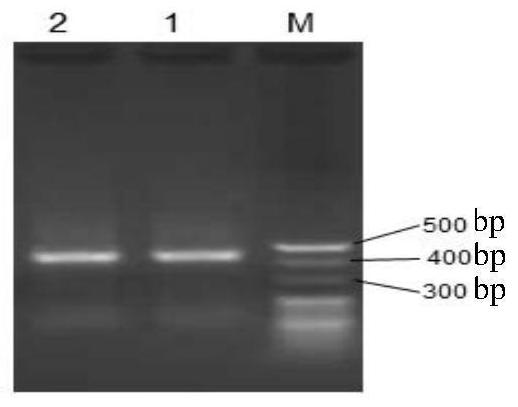
[0121] Replace the DNA fragment between the EcoR I and Xba I recognition sequences of the pGAPZα A vector with the CAMPILs gene shown in Sequence 2, keep the other sequences of the carrier unchanged, and obtain a recombinant vector, which is named pGAPZα A-P (abbreviated as pG- P). pG-P can express antimicrobial peptides (CAMPILs) shown in sequence 1.
[0122] Use AvrII to digest pG-P to obtain linearized pG-P; introduce linearized pG-P into Pichia pastoris SMD1168, and screen to obtain recombinant yeast with linearized pG-P inserted in the genome. The recombinant yeast is named SMDpG-...
Embodiment 2
[0160] Embodiment 2, the biological activity research of SMDpG-P fermentation product in mice
[0161] 1. Preparation of fermentation products
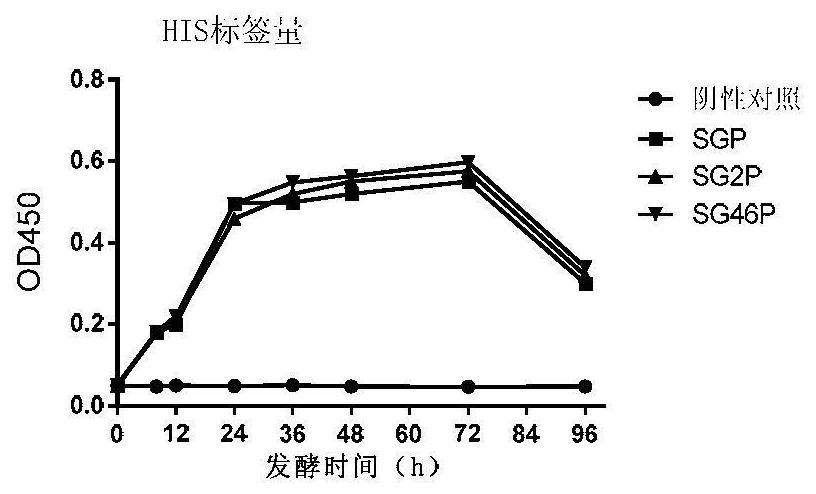
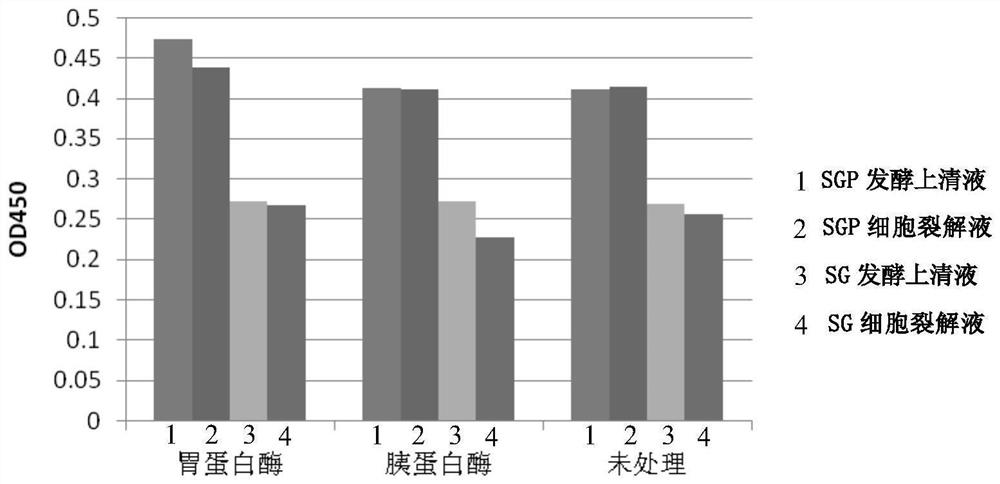
[0162] The SMDpG-P (hereinafter referred to as SGP) of Step 1 of Example 1 was activated and inoculated in a 100mL Erlenmeyer flask containing 30mL of YPD medium, and cultivated at 30°C and 220rpm for 48h to make the OD 600 About 25 to obtain the SGP fermented liquid.
[0163] According to the above method, the SMDpG-P was replaced by the SMDpG (hereinafter referred to as SG) in the first step of Example 1, and the other steps were all unchanged to obtain the SG fermentation broth.
[0164] 2. Grouping of experimental ICR mice
[0165] Take 80 18-20g 3-week-old healthy female ICR mice, randomly divide them into 8 groups, 10 mice in each group, and the group numbers are 1-8, of which Group 1, Group 4, and Group 7 are SG negative control groups, Groups 3 and 6 are vaccine negative control groups, and groups 2, 5 and 8 are experimenta...
Embodiment 3
[0189] Embodiment 3, the biological activity research of SMDpG-P fermentation product in piglet body
[0190] 1. Preparation of fermentation products
[0191] The SMDpG-P (hereinafter referred to as SGP) of Step 1 of Example 1 was activated and inoculated in a 100mL Erlenmeyer flask containing 30mL of YPD medium, and cultivated at 30°C and 220rpm for 48h to make the OD 600 About 40, obtain the SGP fermented liquid.
[0192] According to the above method, the SMDpG-P was replaced by the SMDpG (hereinafter referred to as SG) in the first step of Example 1, and the other steps were all unchanged to obtain the SG fermentation broth.
[0193] 2. Experimental animals were fed fermentation broth
[0194] Seventeen 45-day-old healthy Tibetan pigs weighing about 8 kg were selected from the Jianyang Base of Sichuan Breeding Pig Performance Testing Center, and randomly divided into the experimental group (9 pigs) and the control group (8 pigs). Each pig in the experimental group was f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com