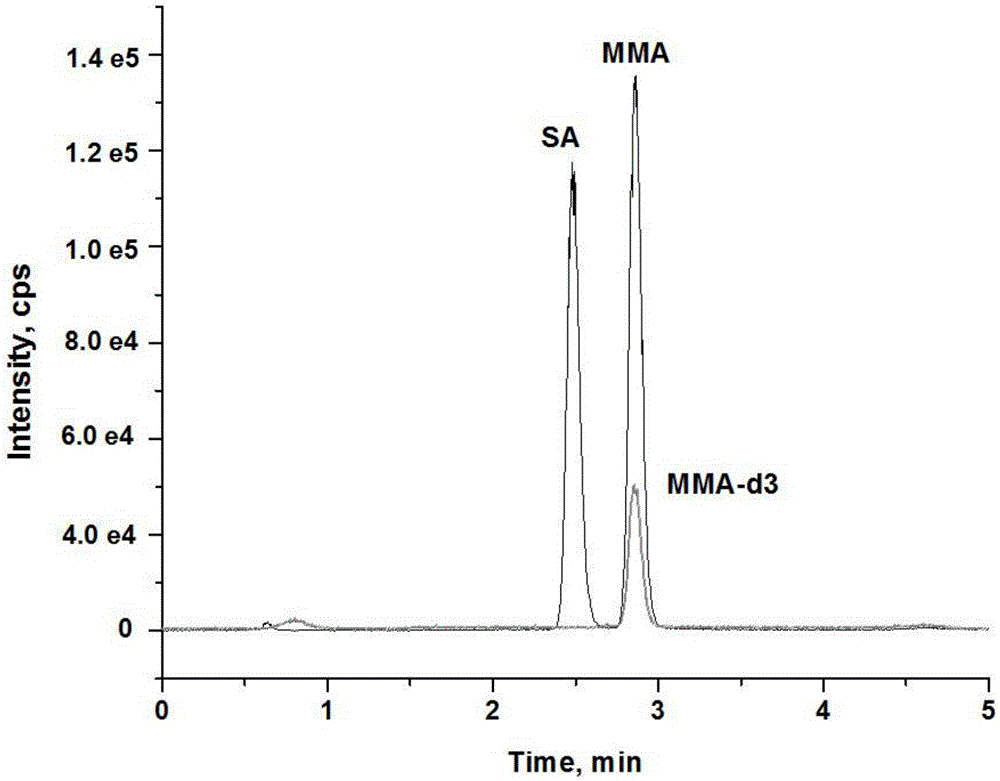
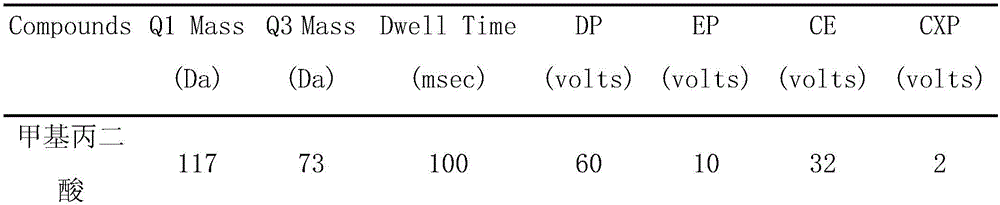
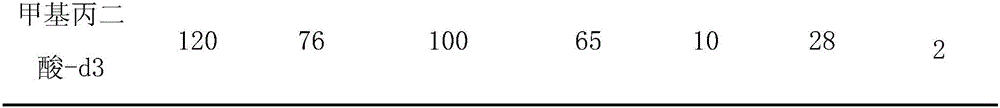Method and kit for detecting methylmalonic acid in blood plasma through high-performance liquid chromatography-tandem mass spectrometry
A high-performance liquid chromatography, methylmalonic acid technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., to achieve the effect of good reproducibility, high accuracy and saving analysis costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1: instrument, material and reagent
[0068] Tandem mass spectrometer (AB Sciex API 5500), high performance liquid phase (Shimadzu LC-20AD XR system). Methylmalonic acid, succinic acid, ammonium acetate and acetic acid were all products from Sigma. Methylmalonate-d3 was purchased from Cambridge Isotope Laboratories. Charcoal-adsorbed serum was purchased from Equitech-Bio, Inc. (USA). HPLC grade ethanol was purchased from J.T. Baker Company, mass spectrometry grade acetonitrile was purchased from Merck Company, 15-mL centrifuge tubes were purchased from Kirgen Company, and volumetric flasks were purchased from BRAND GMBH+CO KG (Germany). Pure water was purified by Millipore Simplicity UA water purifier.
Embodiment 2
[0069] Embodiment 2: the preparation method of calibrator and internal standard solution, comprises the following steps:
[0070] (1) Preparation of standard mother liquor
[0071] Accurately weigh 12.5mg of methylmalonic acid standard substance, dissolve it with pure water and make it to 50mL to obtain a stock solution of 0.25mg / mL (2.1mmol / L), and dilute it successively with pure water to obtain the standard substance mother solution I: 21μmol / L L and standard stock solution II: 100 μmol / L.
[0072] (2) Preparation of standard products
[0073] Preferably, the mother liquor of the standard is diluted with a diluent to obtain 6 different concentrations of the calibrator: 0.05, 0.10, 0.25, 0.50, 1.0, 2.0 μmol / L methylmalonic acid. Each 1 mL of the calibrator with different concentrations was stored at -20°C.
[0074] (3) Preparation of internal standard solution methylmalonic acid-d3 aqueous solution
[0075] Weigh methylmalonic acid-d3, dissolve it in ultrapure water, and...
Embodiment 3
[0076] Embodiment 3: Determination of the plasma sample pretreatment process, including the following steps:
[0077] Take 200 μL plasma into a 2 mL centrifuge tube, add urease, incubate at 37°C for 30 minutes to remove urea, add 20 μL internal standard and 800 μL absolute ethanol, centrifuge at 10,000 rpm for 5 minutes to remove protein, take supernatant, 50 Blow dry with high-purity nitrogen at ℃, redissolve in 500 μL 80% acetonitrile water, vortex for 3 minutes; centrifuge at 13000 r / min for 3 minutes, and inject for detection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com