Reduction-responding polyethylene glycol-polycarbonate targeting maytansine prodrug micelle and preparation method and application thereof
A technology of polyethylene glycol and polycarbonate, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. Problems such as slow release of cancer drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Embodiment one polymer (PEG-P(TMC- co- PDSC)) synthesis
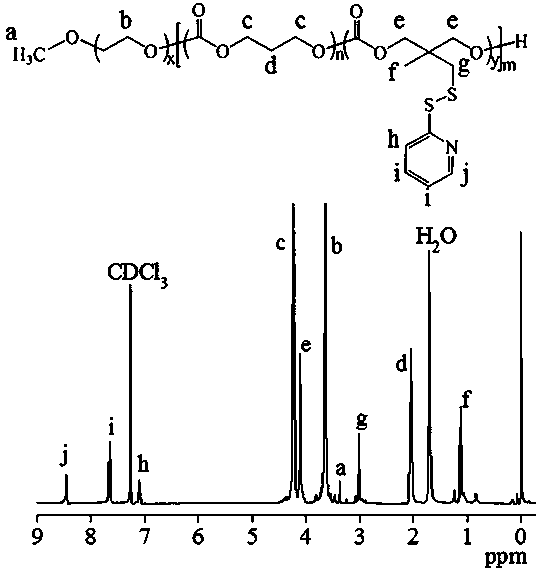
[0087] Under a nitrogen atmosphere, 86.7 mg (0.32 mmol) of dithiopyridine carbonate monomer (PDSC) and 81.6 mg (0.8 mmol) of trimethylene carbonate (TMC) were dissolved in 2 mL of dichloromethane and added to a sealed reactor Li, then add 0.1 g (0.02 mmol) CH 3 O-PEG-OH (5 K) and 0.5 mL catalyst bis(bistrimethylsilyl)amine zinc in dichloromethane solution (0.1 mol / L), seal the reactor, transfer out of the glove box, 40 ℃ oil After reacting in the bath for 24 hours, glacial acetic acid terminated the reaction, precipitated in glacial ether, and finally filtered and vacuum-dried to obtain the polymer PEG-P (TMC- co- PDSC). The yield was 81.5%. The NMR picture is attached figure 1 , 1 H NMR (400 MHz, CDCl 3 ): TMC moieties: δ (ppm) 2.05, 4.23, PEG moieties: δ (ppm) 3.37, 3.65, PDSC moieties: δ (ppm) 1.12, 3.01, 4.11, 7.09, 7.65, 8.46. According to NMR calculation, the molecular weight is 11.8 kg / mol, and ...
Embodiment 2
[0091] Embodiment two amphiphilic polyethylene glycol-polycarbonate maytansinoid prodrug polymer (PEG-P(TMC- g -SSDM1)) synthesis
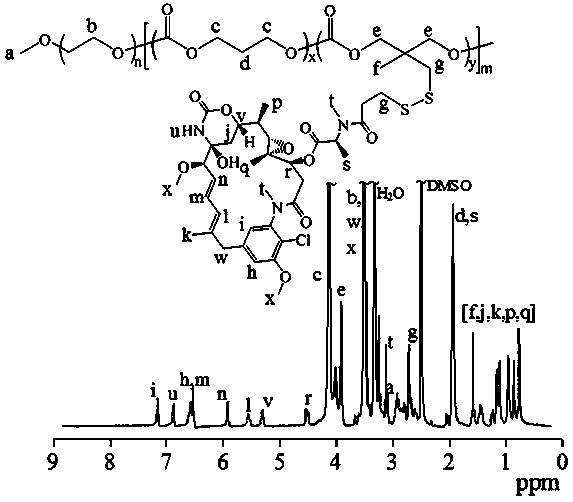
[0092] Under the protection of nitrogen, the polymer PEG-P (TMC- co- PDSC) (100 mg, 0.088 mmol dithiopyridine functional group), while adding a catalytic amount (100 μL) of glacial acetic acid, and then adding 25 mL of thiol-functionalized maytansine (DM1) (97.8 mg, 0.135 mmol) in DMF, the reactor was placed in an oil bath at 35°C, stirred for 48 hours, then dialyzed in DMF and water (Spectra / Pore, MWCO 7000), and freeze-dried with a yield of 75%. NMR results showed that its structure was amphiphilic polyethylene glycol-polycarbonate maytansine prodrug polymer, and the drug loading capacity of maytansine was 40 wt .%. The NMR picture is attached figure 2 , 1 H NMR (600 MHz, DMSO- d 6 ): PEG: δ 3.37, 3.64; TMC: δ 4.24, 2.04; DM1: δ 5.22-7.24, 0.71-1.52, and 3.25-3.55. The NMR calculation gave a molecular weight of 18.1 kg / mol.
Embodiment 3
[0093] Example three polymers (Mal-PEG-P(TMC- co- PDSC)) synthesis
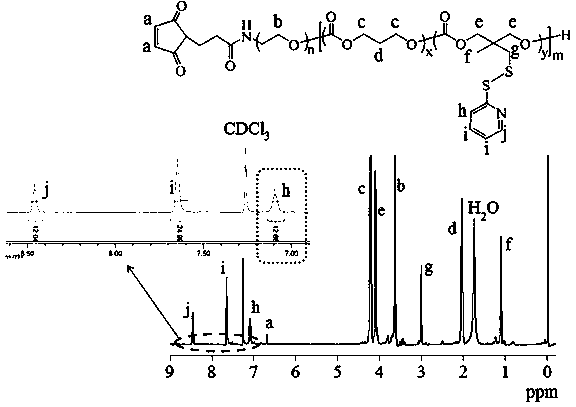
[0094] Under a nitrogen atmosphere, 86.7 mg (0.32 mmol) of dithiopyridine carbonate monomer (PDSC) and 81.6 mg (0.8 mmol) of trimethylene carbonate (TMC) were dissolved in 2 mL of dichloromethane and added to a sealed reactor Then add 0.1 g (0.02 mmol) MAL-PEG-OH (5 K) and 0.5 mL catalyst bis(bistrimethylsilyl)amine zinc in dichloromethane solution (0.1 mol / L), seal the reactor , transferred out of the glove box, reacted in an oil bath at 40°C for 24 hours, terminated the reaction with glacial acetic acid, precipitated in glacial ether, and finally obtained the polymer MAL-PEG-P (TMC- co- PDSC). The yield was 82.3%. The NMR picture is attached image 3 , 1 H NMR (400 MHz, CDCl 3 ): PEG moieties: δ 3.63; TMC moieties: δ4.23, 2.05; PDSC moieties: δ 8.46, 7.65, 7.09, 4.10, 3.01, 1.12, Maleimidegroup: δ 6.71. According to NMR calculation, the molecular weight is 12 kg / mol, and the degrees of polymerizati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com