Absorbable iron-based alloy implantation medical appliance
A technology for implanting medical devices and iron-based alloys, which is applied in medical science, pharmaceutical formulations, coatings, etc., can solve the problems of increasing the risk of inflammatory reactions, incomplete skinning, and fractures, so as to reduce degradation consumption and inflammation risk, the effect of increasing utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
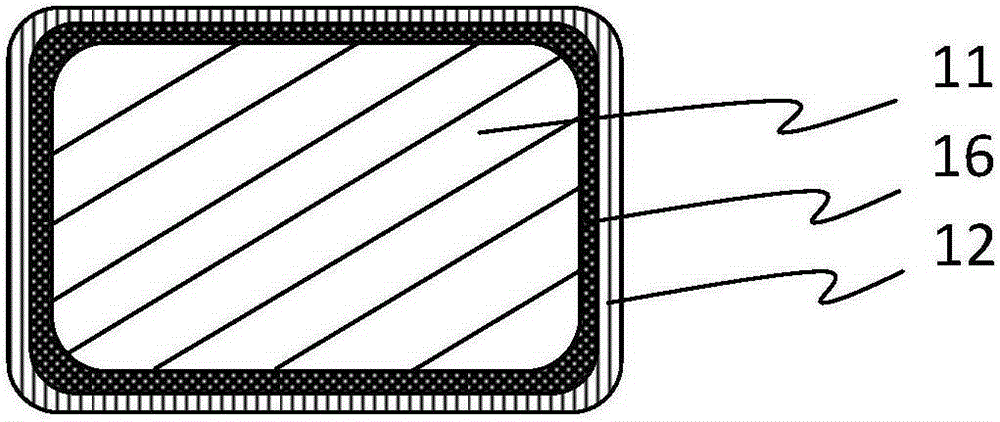
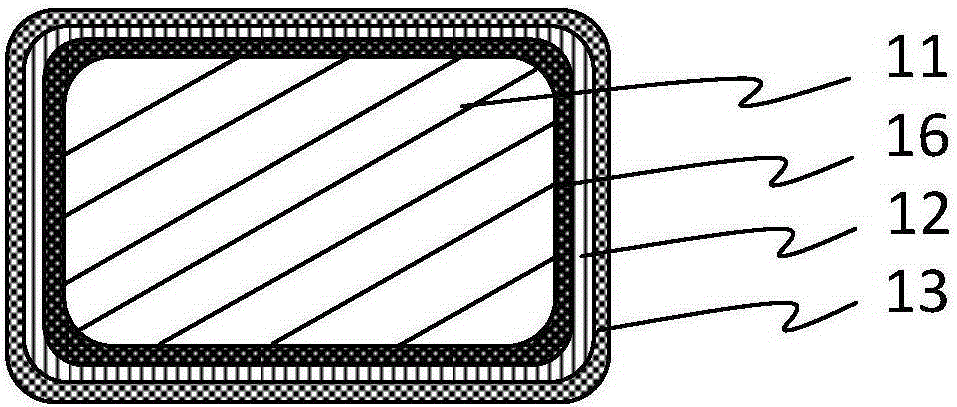
[0044] Select 30008 specifications of iron-based alloy coronary stent after polishing and nitriding treatment, its original radial strength is 145kPa, and its mass is 4.5mg, spray polyracemic lactic acid-ethyl acetate solution on the surface of the stent matrix, and dry it to obtain A degradable poly(racemic lactic acid) coating with a thickness of 6 μm that completely covers the surface of the stent; the surface of the stent prepared in the above steps is sprayed with octacosanol-chloroform solution to form a thickness that completely covers the degradable poly(racemic) lactic acid coating 2 μm octacosanol corrosion inhibition layer. The iron-based absorbable stent prepared by the above steps was implanted into the abdominal aorta of rabbits and removed after 3 months. The endothelialization of the stent was complete without early thrombosis and inflammation. The radial support strength was 80kPa, and the same group of animal experiments monitored complete corrosion. The cycl...
Embodiment 2
[0046] Get the iron-based alloy coronary stent matrix after nitriding treatment of 30008 specification polishing, its original radial strength 145kPa, weight 4.5mg, spray polyracemic lactic acid-ethyl acetate solution on the surface of the stent matrix, make completely after drying A degradable poly(racemic lactic acid) coating with a thickness of 10 μm covering the surface of the stent; the surface of the stent prepared in the above steps is sprayed with glyceryl tristearate-chloroform solution to form a layer that completely covers the degradable poly(racemic lactic acid) coating. Glyceryl tristearate corrosion inhibition layer with a thickness of 2 μm. The iron-based absorbable stent prepared by the above steps was implanted into the abdominal aorta of rabbits and removed after 3 months. The endothelialization of the stent was complete without early thrombosis and inflammation. The radial support strength was 80kPa, and the same group of animal experiments monitored complete...
Embodiment 3
[0048] Get the polished nitrided iron-based alloy coronary stent matrix of 30008 specifications, its original radial strength is 145kPa, and its mass is 4.5mg, spray polyracemic lactic acid-ethyl acetate solution on the surface of the stent matrix, and make it completely covered in The thickness of the stent surface is a 6 μm degradable poly-dl-lactic acid coating; the surface of the stent prepared in the above steps is sprayed with octacosanol-chloroform solution to form a fully covered degradable poly-dl-lactic acid coating with a thickness of 5 μm Octacosanol corrosion inhibitor layer. The absorbable iron-based stent prepared by the above steps was implanted into the abdominal aorta of rabbits and removed after 3 months. The endothelialization of the stent was complete without early thrombosis and inflammation. The radial support strength was 110kPa, and the same group of animal experiments monitored complete corrosion. The cycle is 18 months.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com