Preparation method and application of thiomethylphenol derivatives based on dual catalytic system
A technology of thiomethylphenol and derivatives, which is applied in the field of preparation of thiomethylphenol derivatives based on a dual-catalysis system, can solve the problems of difficult acquisition or preparation of catalysts, difficulty in adapting to industrial production, and excessive generation of "three wastes", achieving The effect of controlling pollutant discharge, easy access, and reducing pollutant discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] One aspect of the present invention provides a method for the preparation of thiomethylphenol derivatives based on a dual catalyst system, which may include: reacting phenol, aldehyde and mercaptan in the presence of a porous material as a catalyst and an amine, After post-treatment to obtain sulfur methyl phenol derivatives.
[0019] In some embodiments, the preparation method specifically includes: mixing phenol, aldehyde, mercaptan, solvent that may or may not be added, porous material, and amine, reacting in a closed environment, and then post-processing to obtain Thiomethylphenol derivatives.
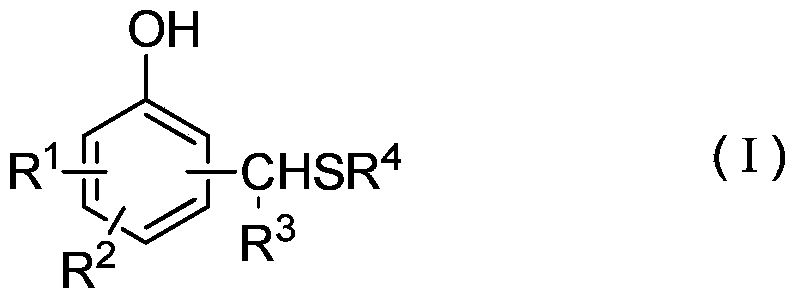
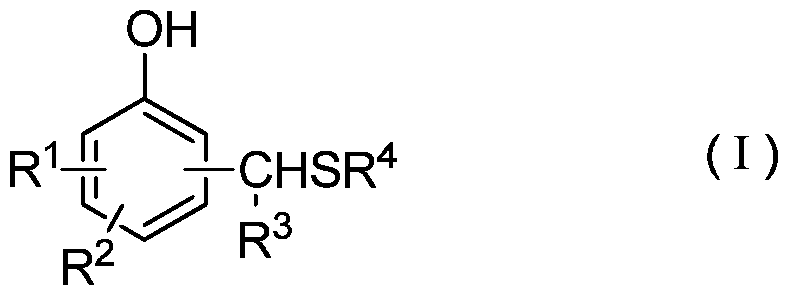
[0020] Wherein, the structure of the thiomethylphenol derivative is shown in the following formula (I):
[0021]
[0022] where R 1 , R 2 , R 3 Respectively represent H, C1~C20 straight chain or branched alkyl or benzene ring substituents or alkyl or aryl containing O, S, N, P and / or Si, R 4 Represents a C1-C20 straight-chain or branched alkyl group or a benzene ring...
Embodiment 1
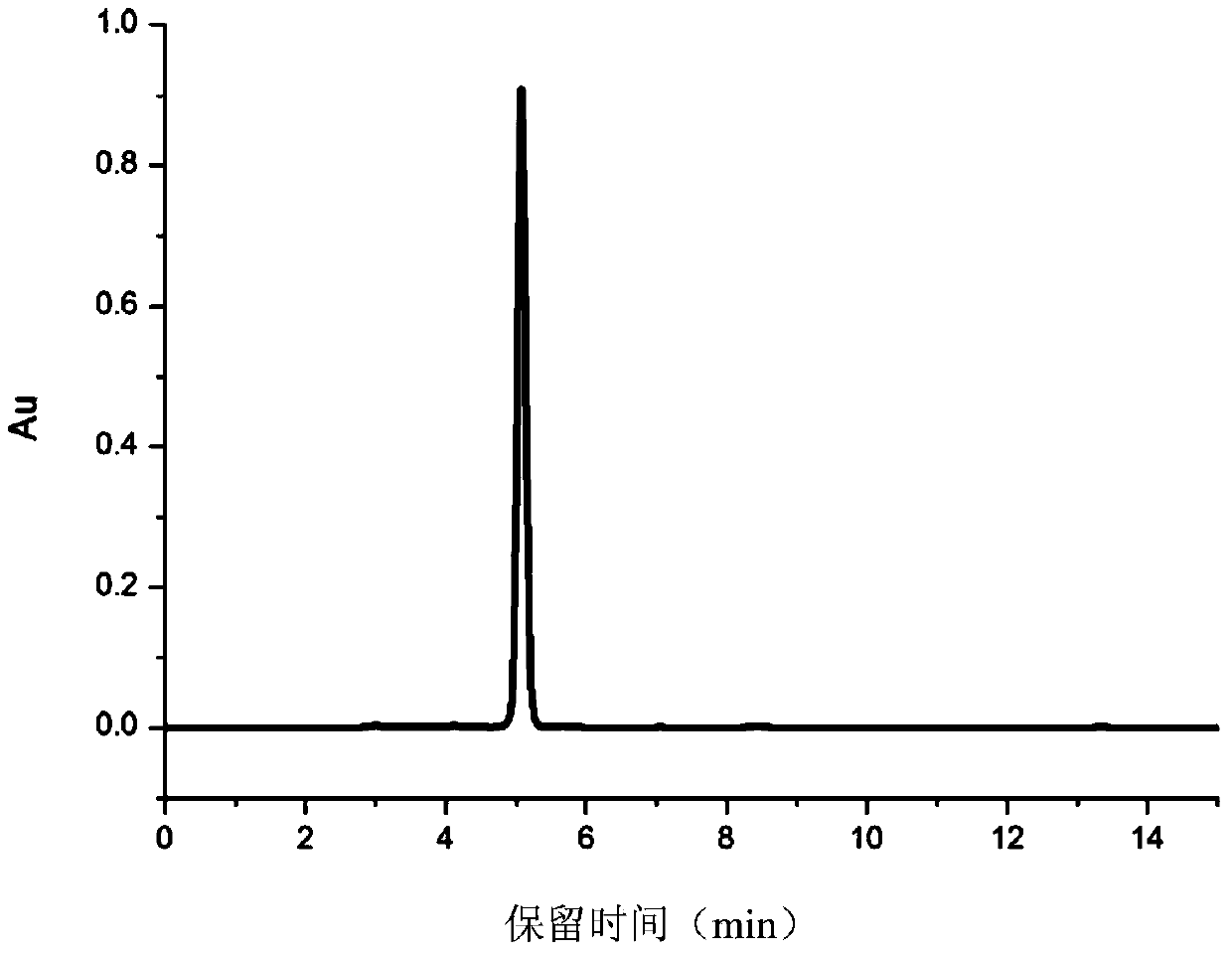
[0052] Example 1 Dissolve 2.0-2.5g of 2,6-di-tert-butylphenol, 0.5-1.0g of paraformaldehyde, 1.0-1.5g of methyl thioglycolate, 50-100mg of diethylamine and 1-3g of montmorillonite in Add about 5mL of DMF into a thick-walled pressure-resistant glass bottle with a magnet, and react for 3-8 hours at a reaction temperature of 50-110°C. After the reaction is complete, cool the reaction mixture to room temperature and filter with suction Isolate the catalyst to obtain the reaction crude product solution, and then remove the solvent and amine under reduced pressure to obtain the target product (3,5-di-tert-butyl-4-hydroxyl-)methyl benzyl mercaptoacetate, and its characterization data are as follows : 1 HNMR (CDCl 3 ):7.11(s,2H,C 6 h 2 ), 5.17(s,1H,Ph-OH),3.76(s,2H,PhCH 2 S),3.73(s,3H,-OCH 3 ),3.12(s,2H,PhCH 2 SCH 2 ),1.44(s,18H,C(CH 3 ) 3 ). Present embodiment product yield is more than 90% on average, and preferred product yield is greater than 95%, and product purity is g...
Embodiment 2
[0053] Example 2 Dissolve 1.0-1.5g of 2,6-dimethylphenol, 1.5-2.0g of formaldehyde solution (37%), 1.5-2.0g of n-octyl mercaptan, 50-100mg of dibutylamine and 1-2g of zeolite Add about 5mL DMSO into a thick-walled pressure-resistant glass bottle with a magnet, and react at a reaction temperature of 140-200°C for 1-5 hours. After the reaction, cool the reaction mixture to room temperature and pump The catalyst is separated by filtration to obtain a crude reaction product solution, and the target product can be obtained by removing the solvent under reduced pressure. The product yield is greater than 90%, and the product purity is greater than 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com