Fluorescent probe capable of separately detecting cysteine/homocysteine, glutathione and sulfuretted hydrogen and preparation method and application of fluorescent probe
A homocysteine and glutathione technology, applied in chemical instruments and methods, fluorescence/phosphorescence, analytical materials, etc., can solve the problems of indistinguishable detection and achieve high yield and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Synthesis of compound 2:
[0036]
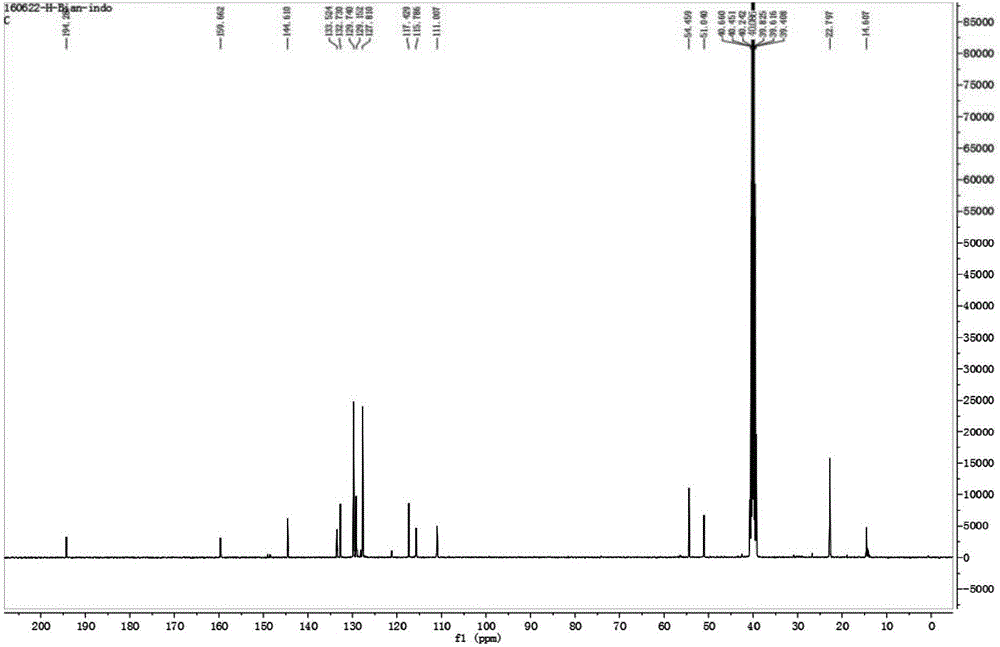
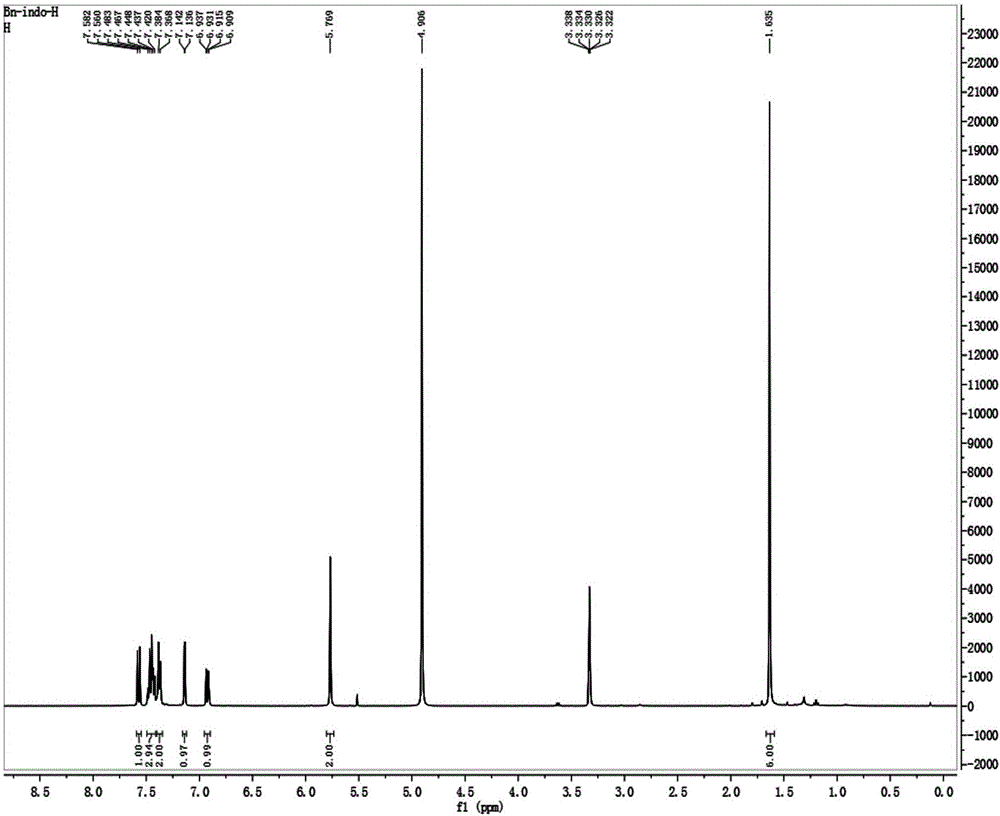
[0037] Place 175mg of oxindole (1.0mmol), 342mg (2.0mmol) of benzyl bromide and 232mg of potassium iodide (2.0mmol) in a round bottom flask, add acetonitrile to completely dissolve the reactant, heat the reaction solution to reflux, and stir for reaction 6 Hour. After the reaction was completed, the solvent was removed by rotary evaporator underpressure distillation to obtain a crude product, which was purified with a silica gel (200-300 mesh) chromatographic column with a volume ratio of 50:1 of dichloromethane and ethanol as the eluent. 252 mg of a dark brown solid were obtained (64% yield). 1 H NMR (400MHz, CD 3 OD), δ(ppm): 1.64(s, 6H), 5.77(s, 2H), 6.91-6.94(dd, J=8.8, 2.4Hz, 1H), 7.136-7.142(d, J=2.4Hz, 1H ), 7.37-7.38(2H), 7.42-7.48(3H), 7.56-7.58(d, J=8.8Hz, 1H); 13 C NMR (100MHz, CD 3 OD): δ(ppm): 14.61, 22.80, 51.04, 54.46, 111.01, 115.79, 117.43, 127.81, 129.15, 129.74, 132.73, 133.52, 144.61, 159.66, 197.29.
[00...
Embodiment 2
[0045] Probe HMN with different equivalents of Cys / Hcy, GSH and H 2 Fluorescence Spectrum Changes of S Reaction
[0046] The probe HMN prepared in Example 1 was dissolved in acetonitrile to make a concentration of 1.0mmol / L probe mother solution (the concentration of probe HMN was 1.0mmol / L); Cys / Hcy, GSH and H 2 S was added to distilled water to prepare Cys / Hcy, GSH and H at a concentration of 5 mmol / L. 2 S mother liquor. Take 30μL from the probe mother solution and add it to a 5mL centrifuge tube, and add different equivalents of Cys / Hcy, GSH (0-100eq) and H 2 S (0-500eq) mother liquor was diluted to 3mL with 0.57mL acetonitrile and different volumes of PBS aqueous solution (concentration 25mmol / L, pH 7.4), and configured as a test solution with a probe concentration of 10μmol / L and 20% acetonitrile. Test the probe with different equivalents of Cys / Hcy, GSH (0-100eq) and H with a fluorescence spectrometer 2 Fluorescence spectrum changes of S (0-500eq) reaction solution (...
Embodiment 3
[0048] Probe HMN and Cys / Hcy, GSH and H 2 Fluorescence change of S over time
[0049] Take 30 μL from the mother solution of the probe in Example 2 and add it to a 5mL centrifuge tube, and add different equivalents of Cys / Hcy (55eq), GSH (65eq) and H 2 S (50 or 500eq) mother solution was diluted to 3mL with 0.57mL acetonitrile and different volumes of PBS aqueous solution (concentration 25mmol / L, pH 7.4), and configured as a test solution with a probe concentration of 10μmol / L and 20% acetonitrile. Using excitation wavelengths of 410, 470 and 550 nm, respectively, the fluorescence spectra of the probes as a function of time were tested. Depend on Figure 8 It can be seen that with the increase of time, after adding Cys / Hcy, the fluorescence intensity at 485 and 609nm gradually increased, and reached the maximum at about 12 / 20 and 15 / 20min respectively; after adding GSH, the fluorescence intensity at 609nm Gradually become larger and reach the maximum value at about 20min; a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com