Cis-tristilbene and its preparation method and detection method
A technique of tristilbene glycoside and detection method, which is applied in the field of medicine and can solve the problems of blank research on impurities and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
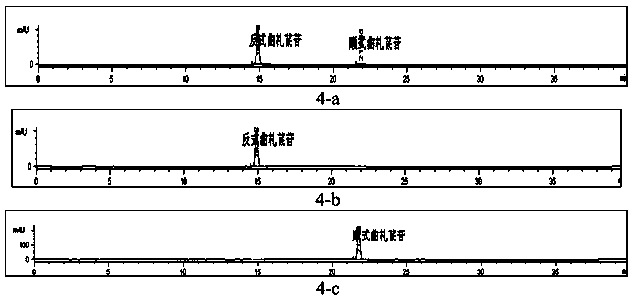
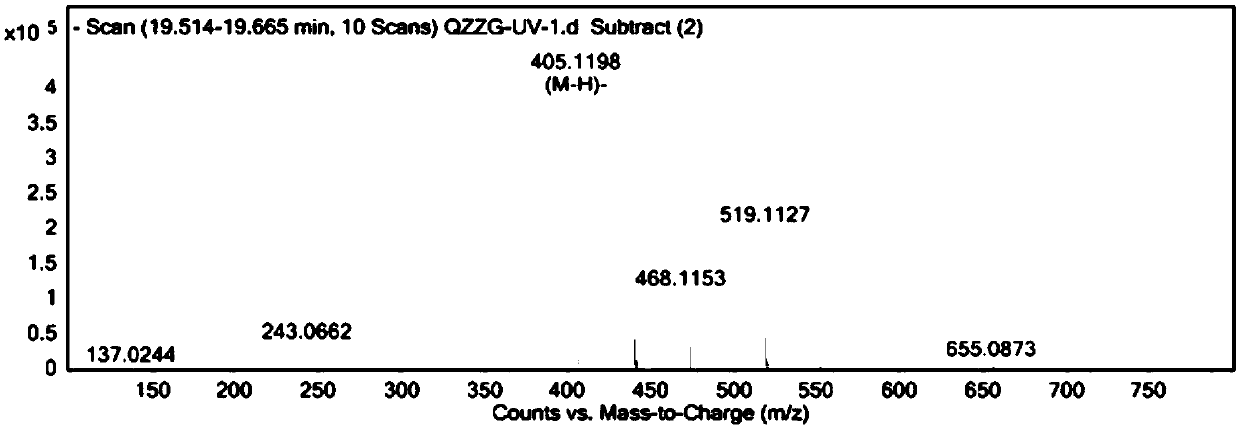
[0080] Example 1: Separation and purification of cis-traza stilbene
[0081] 1. Main instruments
[0082] Agilent 1100 high performance liquid chromatograph (including online degasser, quaternary pump, autosampler, VWD detector) (Agilent), Bruker Avance 800M nuclear magnetic resonance instrument (Bruker), CP225D analytical balance (Sartorius) Company), Milli-Q ultrapure water machine (Millipore company), Agilent QTOF 6540 mass spectrometer (Agilent company), Buchi R-3 rotary evaporator (Buchi company), ED-IE-50 freeze dryer (Shanghai Bilan Instruments Limited).
[0083] 2. Experimental materials
[0084] (1) Reagents: methanol (analytical purity, Tianjin Fengchuan Chemical Reagent Technology Co., Ltd.), ultrapure water, trans-triza stilbene sample (made by Kunyao Group Co., Ltd. Institute of Medicine, containing cis-triza stilbene) Glycoside about 2.0%), deuterated methanol (Sigma-Aldrich company).
[0085] (2) Chromatographic column: Agilent ZORBAX SB-C 18 (250mm×9.4mm, 5μm)
[0086]...
Embodiment 2
[0090] Example 2: Determination of biological activity
[0091] Thrombin is an important factor in the body's coagulation system. It is a multifunctional protease produced in the injured vascular endothelial cells. It is located at the back of the coagulation activation pathway and is an effector that triggers the coagulation reaction. When the vascular intima is damaged, platelets adhere, aggregate, and activate to promote blood coagulation, thereby activating the coagulation system. The prothrombin complex activates thrombin, turning fibrinogen into insoluble fibrin and forming a thrombus. Early experiments showed that trans-traza stilbene has the activity of inhibiting thrombin, which is closely related to its efficacy in preventing and treating ischemic stroke.
[0092] In this experiment, the fluorescence method is used to determine the combination of thrombin and the substrate to expose the fluorescent group in the substrate. The enzyme reaction produces fluorescence at the e...
Embodiment 3
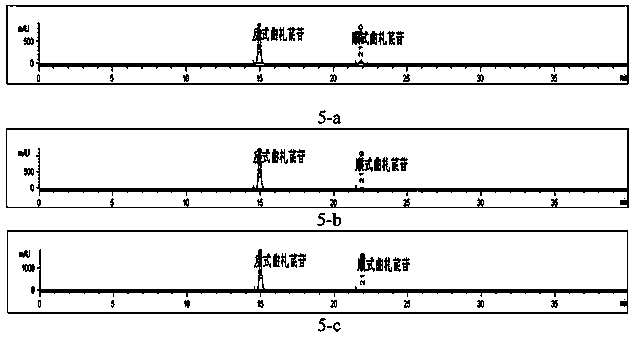
[0114] Example 3: Preparation of cis-traza stilbene
[0115] 1. Main instruments
[0116] Agilent 1100 high performance liquid chromatograph (including online degasser, quaternary pump, autosampler, VWD detector) (Agilent), CP225D analytical balance (Sartorius), Milli-Q ultrapure water machine ( Millipore company), ZF-6 ultraviolet lamp (Shanghai Jiapeng Technology Co., Ltd.), Buchi R-3 rotary evaporator (Buchi company), ED-IE-50 freeze dryer (Shanghai Bilang Instrument Co., Ltd.).
[0117] 2. Experimental materials
[0118] (1) Reagents: methanol (analytical purity, Tianjin Fengchuan Chemical Reagent Technology Co., Ltd.), ultra-pure water, Quza stilbene reference substance (made by Kunyao Group Co., Ltd. Institute of Medicine).
[0119] (2) Chromatographic column: Phenomenex Luna Su C 18 (2) column (250mm×4.60mm, 5μm) 3. Isomerization of trans-traza stilbene
[0120] Take 130 mg of trans-traza stilbene glycoside sample, dissolve it in 50ml methanol, irradiate it under 365nm ultraviole...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com