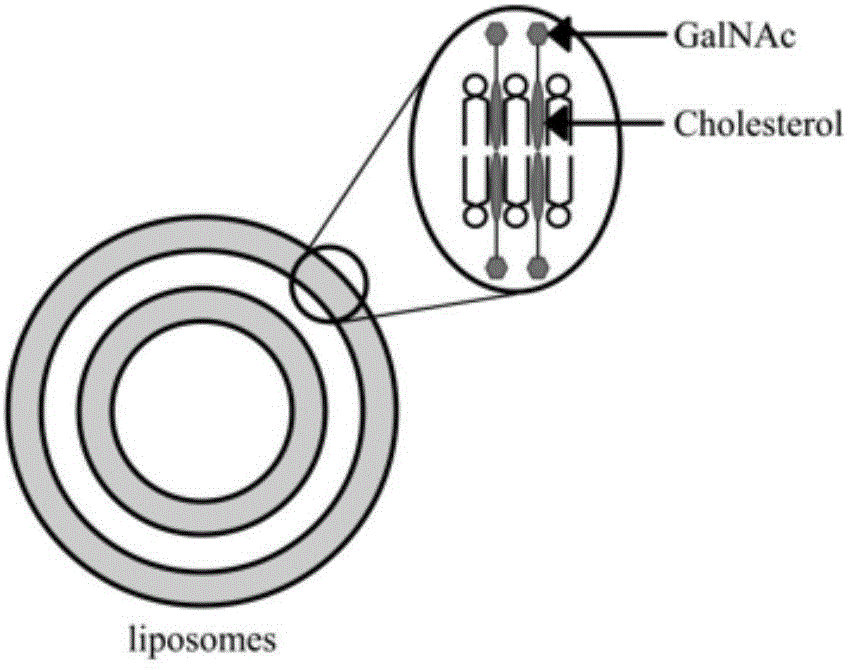
Liver cancer targeting glycoside ligand molecule, preparation method thereof, and drug delivery system
A ligand molecule and drug-loading system technology, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as lengthy steps, complicated reaction conditions, and low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Synthesis of GalNAc-C8-Chol
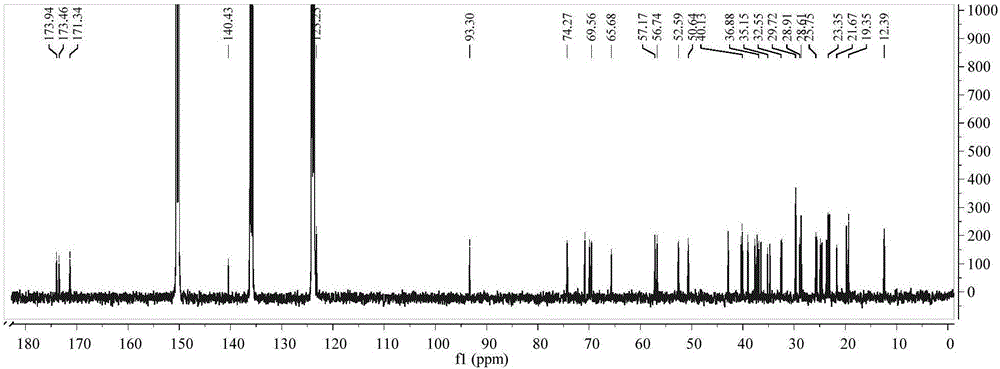
[0036] Method: Take 10mL stoppered vial, add CHS-SE (0.1mmol), GalNAc (0.05mmol), add 5mL dehydrated acetone, add enzyme TL IM 20mg, shake in an air bath constant temperature oscillator (45℃, 250r·min- 1), react for 24h. After the reaction is finished, the enzyme is filtered out, and the solvent is recovered to obtain the product. The reaction equation is shown in figure 1 shown. The product was purified by flash silica gel column chromatography, and the structure of the purified product was identified by infrared, ESI, 1H NMR, and 13C NMR.
[0037]
[0038] Identification of synthetic products
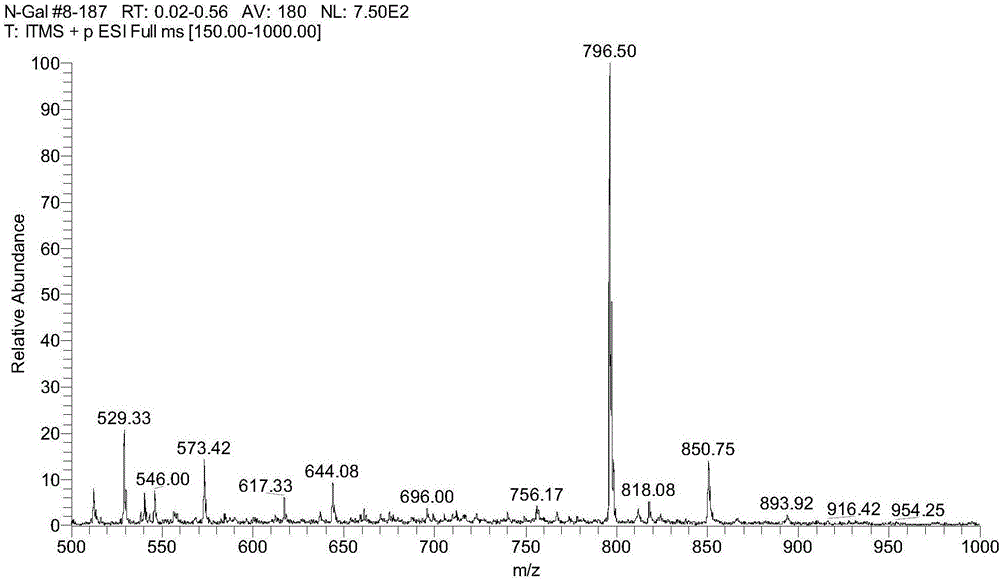
[0039] The structure of the product was identified as the target product by MS and NMR, and the specific data are as follows:
[0040] MS conditions: After the product is dissolved in an appropriate amount of methanol, it is analyzed by a mass spectrometer (MS), mass spectrometry parameters: triple quadrupole LC-MS / MS: electrospray...
Embodiment 2
[0045] Example 2 Preparation and Characterization of GalNAc Modified Nanoliposomes
[0046] Preparation method: Weigh phospholipids (PC), cholesterol (CHS), DSPG-Na, GalNAc ligand molecules, drugs, etc. according to a certain ratio (40:20:1:4:1) and mix and dissolve them in chloroform. Remove chloroform by rotary steaming at 55°C and 40rpm in an eggplant-shaped bottle to form a uniform lipid film, add pH 7.4 PBS buffer solution, hydrate at 55°C for 1 hour, and then squeeze and filter with 100nm and 50nm filter membranes in turn to obtain uniform nanoparticles.
[0047] And the particle size of the two ( Figure 6 ), zeta potential ( Figure 7 ), encapsulation efficiency, etc. Results Using this method, the nanoparticle size was smaller, the particle size distribution was uniform, the quality was stable, and the encapsulation efficiency was high (see Table 1), which met the requirements of subsequent tests.
[0048] The comparison (n=3) of table 1 common liposome and galacto...
Embodiment 3
[0050] Example 3 In vitro targeting studies of GalNAc-modified nanoliposomes
[0051] Experimental method: Human liver cancer HepG2 cells in the logarithmic growth phase were inoculated in a 24-well plate. After the cell confluence reached about 80% and the cell shape was plump, an appropriate amount of common fluorescent liposome (FL-LP) and GalNAc were added respectively. -LP-FL (adjust the lipid concentration to 1mmol·mL with medium -1 ). At 37°C, 5% CO 2 Incubate in the incubator for 1 hour, then use 1mL PBS (pH7.4) to wash away the fluorescent nanoliposomes that were not taken up by the cells, lyse the cells with 1% TriotnX-100PBS, and measure the uptake of FL-LP and GalNAc- by HepG2 cells with a microplate reader. The fluorescence intensity of LP-FL was evaluated in vitro for liver tumor targeting.
[0052] The liver targeting of GalNAc-modified fluorescent liposomes (GalNAc-LP-FL) was evaluated by human liver cancer HepG2 cells, and the results showed that the uptake...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com