Dendritic crystal corroding agent for high nitrogen austenitic stainless steel and preparation method thereof
A technology of high-nitrogen austenite and nitrogen austenite, applied in the field of low-magnification inspection and analysis of steel billets, can solve problems such as poor effect, poor dendrite corrosion effect, long preparation time of etchant, and achieve clear display and reproduction good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
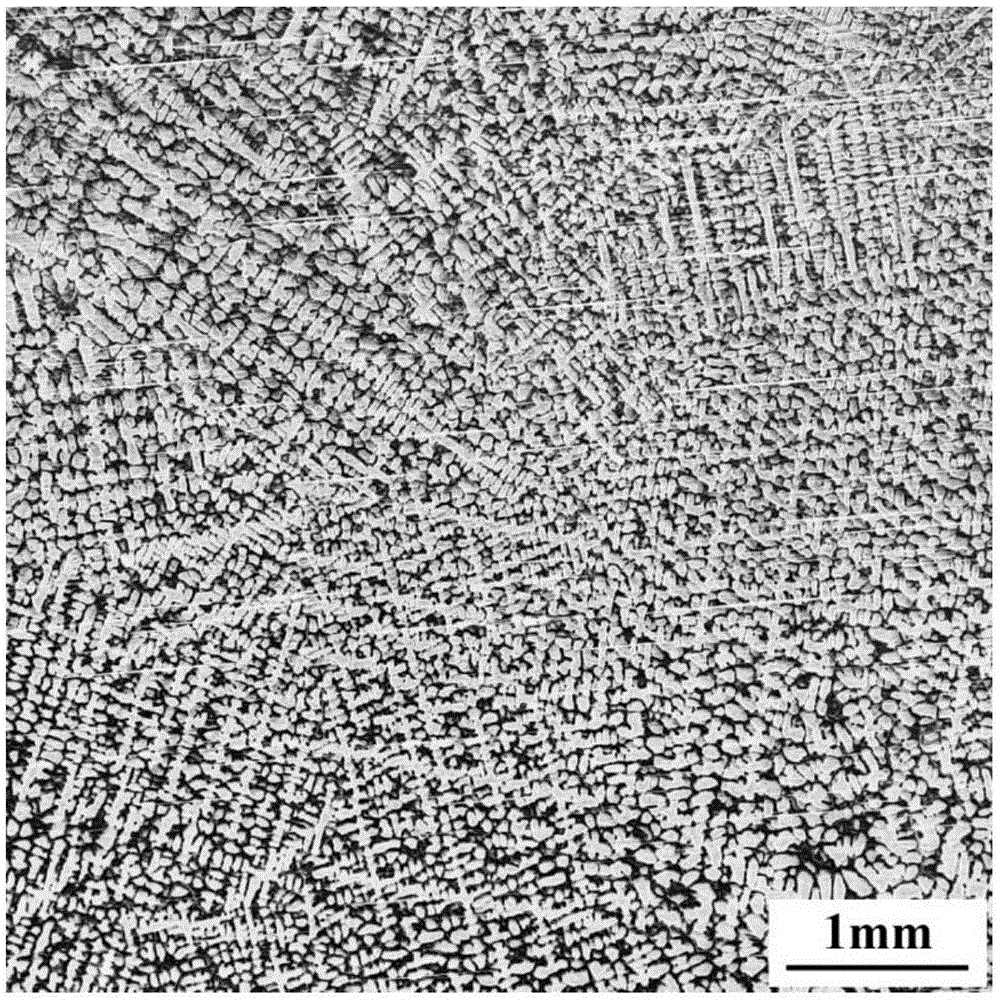
Embodiment 1
[0035] The chemical composition of the high nitrogen austenitic stainless steel sample in this example is shown in Table 1.
[0036] Table 1 Chemical composition of high nitrogen austenitic stainless steel samples (wt%)
[0037]
[0038] A dendritic etchant for high nitrogen austenitic stainless steel, which contains the following components and proportions: 2.01 g of copper chloride, 0.15 g of magnesium chloride, 1.56 g of ferric chloride, 100.0 mL of water, 11.5 mL of hydrochloric acid, and 3.2 mL of nitric acid , anhydrous ethanol 130.0mL;
[0039] Wherein, the hydrochloric acid is concentrated hydrochloric acid with a mass percentage concentration of 36-38%, and the nitric acid is nitric acid with a mass percentage concentration of 65-68%. A method for using a dendritic etchant for high nitrogen austenitic stainless steel, comprising the following steps:
[0040] (1) Preparation of dendritic etchant for high nitrogen austenitic stainless steel
[0041] Add water, cop...
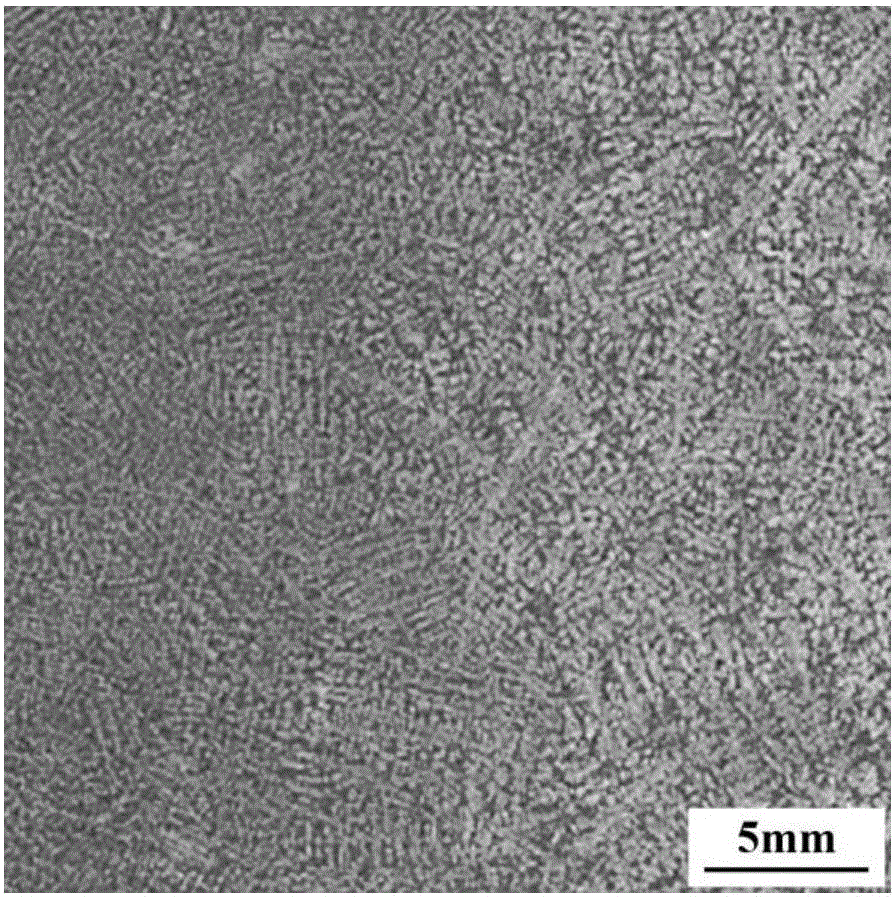
Embodiment 2
[0050] The chemical composition of the high nitrogen austenitic stainless steel sample in this example is shown in Table 2.
[0051] Table 2 Chemical composition of high nitrogen austenitic stainless steel samples (wt%)
[0052]
[0053] A dendritic etchant for high-nitrogen austenitic stainless steel, which contains the following components and proportions: 0.69 g of copper chloride, 0.22 g of magnesium chloride, 2.3 g of ferric chloride, 100.0 mL of water, 9.0 mL of hydrochloric acid, and 1.5 mL of nitric acid , anhydrous ethanol 125.0mL;
[0054] Wherein, hydrochloric acid is concentrated hydrochloric acid whose mass percentage concentration is 36-38%, and nitric acid is nitric acid whose mass percentage concentration is 65-68%
[0055] A method for using a dendritic etchant for high nitrogen austenitic stainless steel, comprising the following steps:
[0056] (1) Preparation of dendritic etchant for high nitrogen austenitic stainless steel
[0057] Add water, copper ...
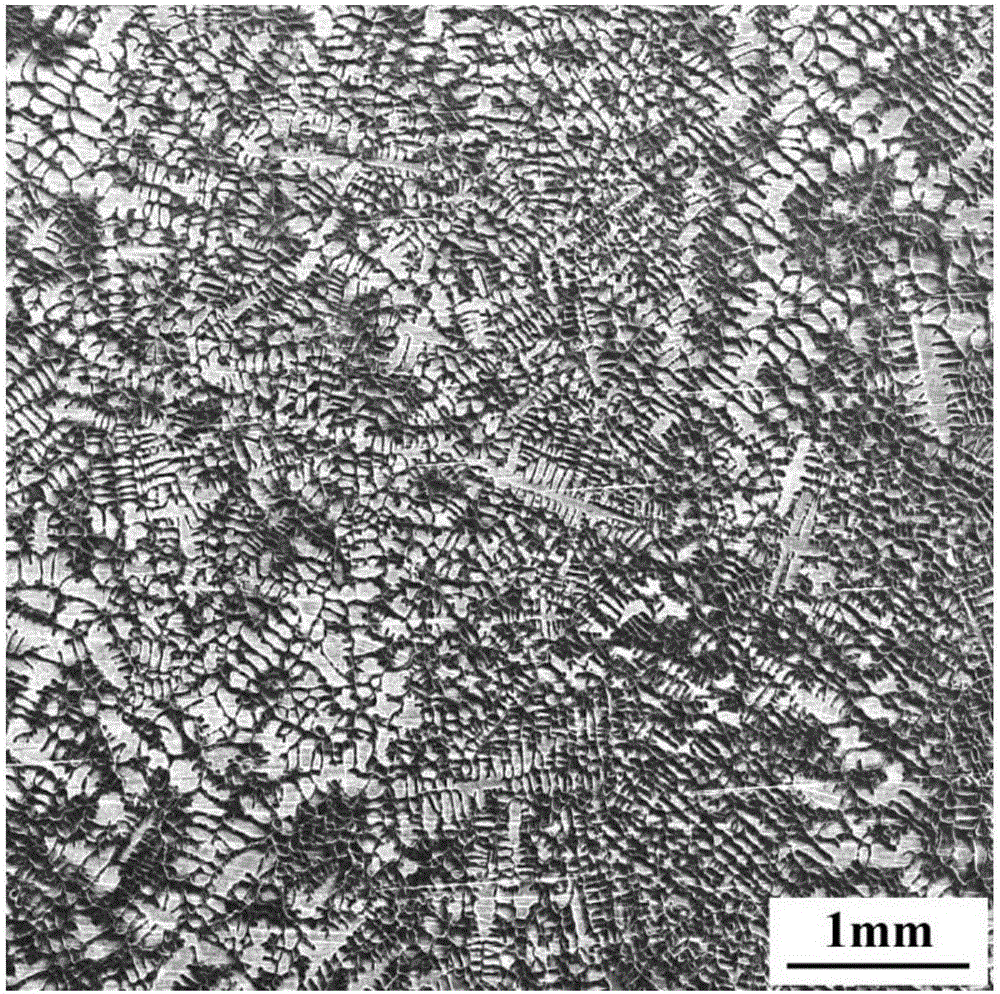
Embodiment 3
[0066] The chemical composition of the high nitrogen austenitic stainless steel sample in this example is shown in Table 3.
[0067] Table 3 Chemical composition of high nitrogen austenitic stainless steel samples (wt%)
[0068]
[0069] A dendritic etchant for high-nitrogen austenitic stainless steel, containing components and proportions as follows: 1.58g of copper chloride, 0.18g of magnesium chloride, 1.95g of ferric chloride, 100.0mL of water, 10.0mL of hydrochloric acid, and 2.5mL of nitric acid , anhydrous ethanol 127.0mL;
[0070] Wherein, hydrochloric acid is concentrated hydrochloric acid whose mass percentage concentration is 36-38%, and nitric acid is nitric acid whose mass percentage concentration is 65-68%
[0071] A method for using a dendritic etchant for high nitrogen austenitic stainless steel, comprising the following steps:
[0072] (1) Preparation of dendritic etchant for high nitrogen austenitic stainless steel
[0073] Add water, copper chloride, m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com