Self-supporting oxygen reduction/oxygen evolution double-effect oxygen electrode catalyst and preparation method therefor
A dual-effect oxygen electrode, self-supporting technology, used in battery electrodes, circuits, electrical components, etc., can solve the problems of inability to large-scale application, lattice changes, poor stability, etc., and achieve excellent oxygen reduction and oxygen evolution performance, good Oxygen evolution performance, effect of good mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
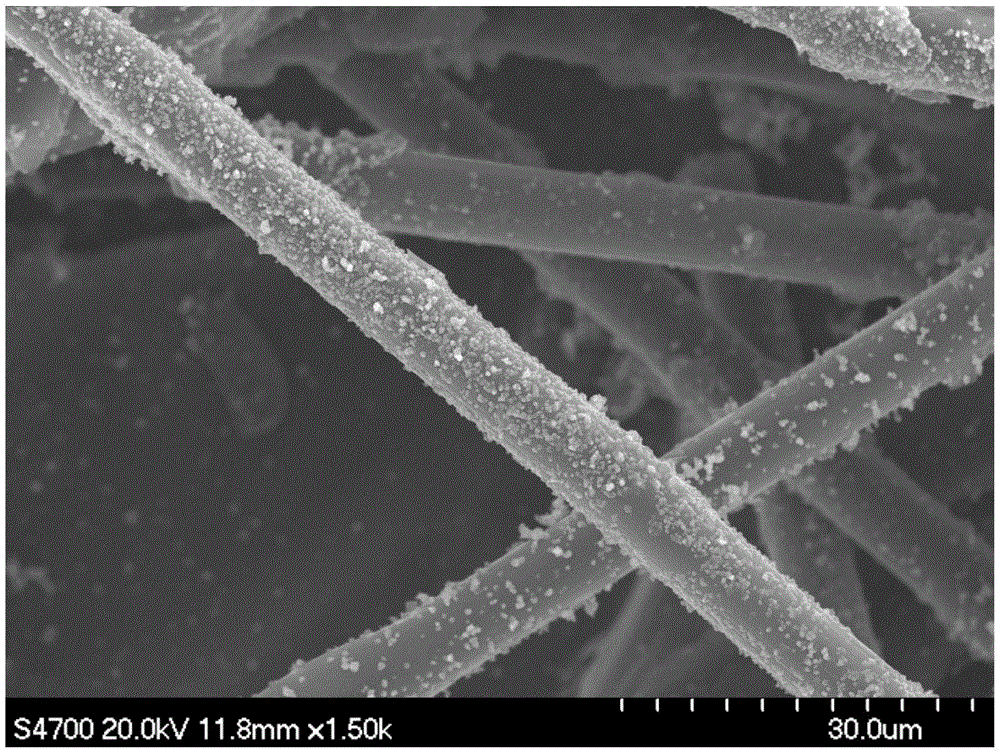
[0035] From the 0.3mm thick hydrophilic carbon paper, cut a small piece of carbon paper (CP) with a length of 12mm and a width of 10mm. Soak a small piece of carbon paper in aqua regia for 6 hours at room temperature, then take out the carbon paper and wash it with a large amount of distilled water.
[0036] 7.6g (100mmol) of thiourea, 0.25g of ferric chloride and 0.284g of nickel acetate tetrahydrate were dissolved in 60ml of deionized water, and stirred evenly to obtain a solution.
[0037] Put the treated carbon paper into a 100ml hydrothermal reaction kettle, pour the above solution into the reaction kettle, react in an oven at 200°C for 12h, and cool naturally after the reaction.
[0038] After cooling, take out the carbon paper, wash it several times with distilled water and ethanol, then soak it in 1mol / L sulfuric acid for 10h, and then wash it with deionized water.
[0039] Put the carbon paper in an oven and dry it at 60°C for 10 hours to obtain carbon paper (Fe-Ni-N...
Embodiment 2
[0041] From the 0.3mm thick hydrophilic carbon paper, cut a small piece of carbon paper with a length of 12mm and a width of 10mm. Soak a small piece of carbon paper in aqua regia for 6 hours at room temperature, then take out the carbon paper and wash it with a large amount of distilled water.
[0042] 7.6g (100mmol) of thiourea, 0.25g of ferric chloride and 0.194g of cobalt chloride were dissolved in 60ml of deionized water, and stirred evenly to obtain a solution.
[0043] Put the treated carbon paper into a 100ml hydrothermal reaction kettle, pour the above solution into the reaction kettle, react in an oven at 180°C for 18h, and cool naturally after the reaction.
[0044] After cooling, take out the carbon paper, wash it several times with distilled water and ethanol, then soak it in 2mol / L hydrochloric acid for 6h, and then wash it with deionized water.
[0045] Put the carbon paper in an oven and dry it at 120°C for 6 hours to obtain carbon paper co-doped with nitrogen...
Embodiment 3
[0047]From the 0.3mm thick hydrophilic carbon paper, cut a small piece of carbon paper with a length of 12mm and a width of 10mm. Soak a small piece of carbon paper in mixed acid (concentrated sulfuric acid: concentrated nitric acid = 3:1) for 1 hour at room temperature, then take out the carbon paper and wash it with a large amount of distilled water.
[0048] 7.6 g (100 mmol) of thiourea and 0.5 g of ferric chloride were dissolved in 60 ml of deionized water, and stirred evenly to obtain a solution.
[0049] Put the treated carbon paper into a 100ml hydrothermal reaction kettle, pour the above solution into the reaction kettle, react in an oven at 180°C for 18h, and cool naturally after the reaction.
[0050] After cooling, take out the carbon paper, wash it several times with distilled water and ethanol, soak it in 2mol / L sulfuric acid for 6h, and then wash it with deionized water.
[0051] Put the carbon paper in an oven and dry it at 100°C for 6 hours to obtain carbon pa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com