A kind of doxylamine succinate hydrochloride pyridoxine enteric-coated tablet medicinal composition and preparation method thereof
A technology of pyridoxine hydrochloride and doxylamine succinate, which is applied in the field of medicine, can solve the problems that the release degree can only reach about 50%, the rapid development of drug effect is affected, and the release is slow, so as to avoid adverse reactions. , low cost, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The preparation of embodiment 1 doxylamine succinate pyridoxine hydrochloride sheet of the present invention
[0047] 1. Tablet core preparation:
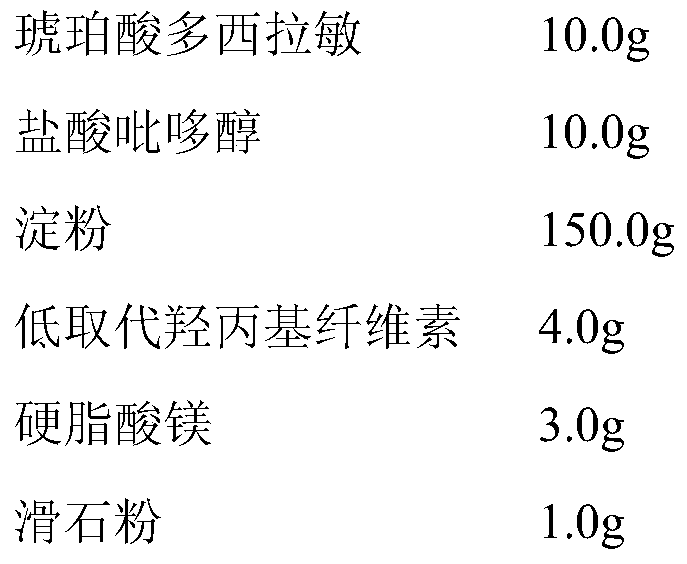
[0048] Prepare raw materials (1000 tablets) according to the following formula:
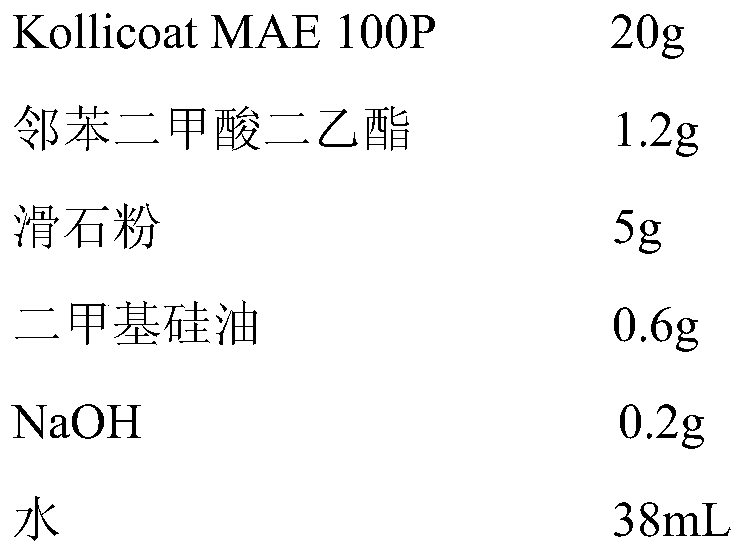
[0049]
[0050] Preparation steps:
[0051] (1) Dry the starch until the water content is less than 1.5%, and dry the talcum powder until the water content is less than 5% at 80°C;
[0052] (2) pulverize doxylamine succinate and pyridoxine hydrochloride, cross 100 mesh sieves, and set aside;
[0053] (3) mix doxylamine succinate and talcum powder, pass through a 40-mesh sieve to disperse, and mix to obtain the mixture ①;
[0054] (4) Pyridoxine hydrochloride is added to the mixture ①, mixed to obtain the mixture ②;
[0055] (5) The low-substituted hydroxypropyl cellulose was mixed twice with starch equivalent dilution method, then added to the mixture ②, and mixed to obtain the mixture ③;
[0056] (6) Add the remaining starch and the mi...
Embodiment 2
[0078] The preparation of embodiment 2 doxylamine succinate pyridoxine hydrochloride tablet of the present invention
[0079] 1. Tablet core preparation:
[0080] Prepare raw materials (1000 tablets) according to the following formula:
[0081]
[0082] Preparation steps:
[0083] (1) Dry the microcrystalline cellulose PH102 until the water content is less than 3%, and dry the silicon dioxide until the water content is less than 5% at 80°C;
[0084] (2) pulverize doxylamine succinate and pyridoxine hydrochloride, cross 100 mesh sieves, and set aside;
[0085] (3) Mix doxylamine succinate with silicon dioxide, disperse through a 40-mesh sieve, and mix to obtain the mixture ①;
[0086] (4) Pyridoxine hydrochloride is added to the mixture ①, mixed to obtain the mixture ②;
[0087] (5) The sodium carboxymethyl starch is mixed twice with mannitol equivalent dilution method and then added to the mixture ②, mixed to obtain the mixture ③;
[0088] (6) Add the remaining mannito...
Embodiment 3
[0110] Embodiment 3 The preparation of doxylamine succinate pyridoxine hydrochloride tablet of the present invention
[0111] 1. Tablet core preparation:
[0112] Prepare raw materials (1000 tablets) according to the following formula:
[0113]
[0114] Preparation steps:
[0115] (1) Dry the microcrystalline cellulose PH102 until the moisture content is less than 3%, dry the lactose until the moisture content is less than 1.5%, and dry the silica until the moisture content is less than 5% at 80°C;
[0116] (2) pulverize doxylamine succinate and pyridoxine hydrochloride, cross 100 mesh sieves, and set aside;
[0117] (3) Mix doxylamine succinate with silicon dioxide, disperse through a 40-mesh sieve, and mix to obtain the mixture ①;
[0118] (4) Pyridoxine hydrochloride is added to the mixture ①, mixed to obtain the mixture ②;
[0119] (5) The croscarmellose sodium was mixed twice with lactose equivalent dilution method, then added to the mixture ②, and mixed to obtain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com