Solid electrolyte material and preparation method therefor, solid electrolyte and battery
A solid electrolyte and electrolyte technology, which is applied in the direction of solid electrolyte, electrolyte battery manufacturing, non-aqueous electrolyte battery, etc., can solve the problems of lower crystallization ratio electrolyte ionic conductivity, restriction of solid electrolyte ionic conductivity, and low ionic conductivity. Achieve excellent charge and discharge performance and cycle performance, excellent ionic conductivity, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The present invention also provides a method for preparing the above-mentioned solid electrolyte material, the method comprising:
[0026] (1) mixing the components that provide the crystalline inorganic solid electrolyte, and then roasting to obtain the crystalline inorganic solid electrolyte;
[0027] (2) The crystalline inorganic solid electrolyte is mixed with a component providing an amorphous inorganic solid electrolyte.
[0028] According to the present invention, step (1) will produce a crystalline inorganic solid electrolyte. Therefore, the present invention has no particular limitation on the components that provide the crystalline inorganic solid electrolyte, as long as it can be used to prepare the crystalline inorganic solid electrolyte. Yes, the batching of the components that provide the crystalline inorganic solid electrolyte makes the resulting crystalline inorganic solid electrolyte have the formula Li 10±1 AB 2 x 12 One or more of the crystalline i...
Embodiment 1
[0047] This example is used to illustrate the solid electrolyte material of the present invention and its preparation method.
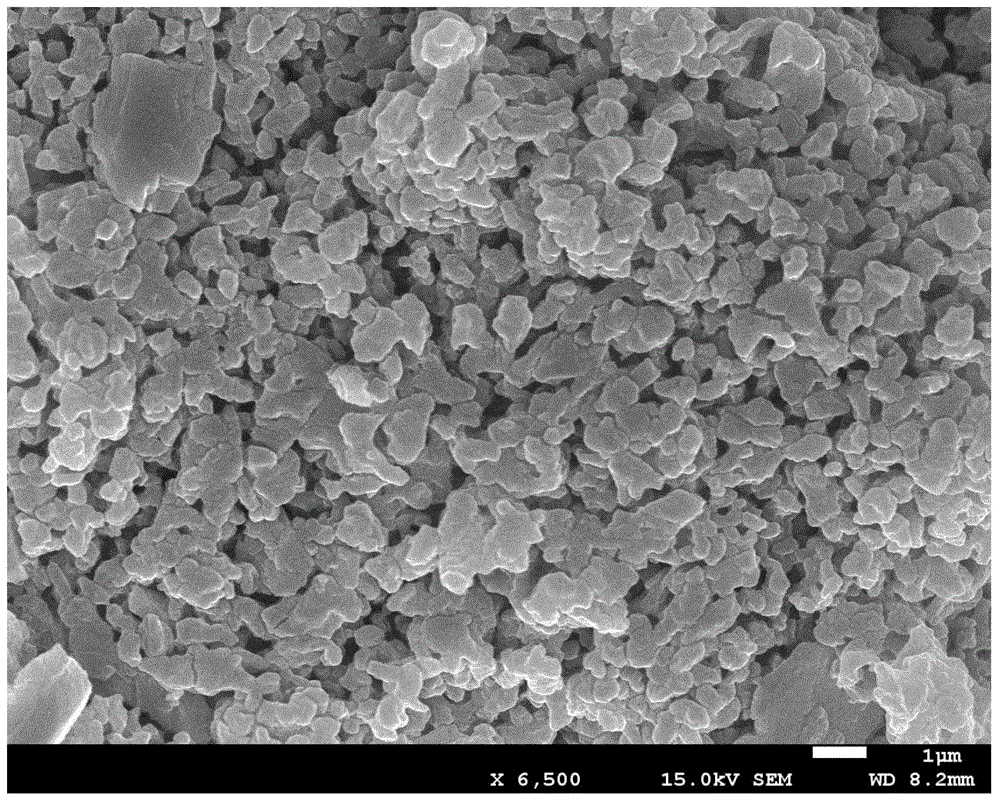
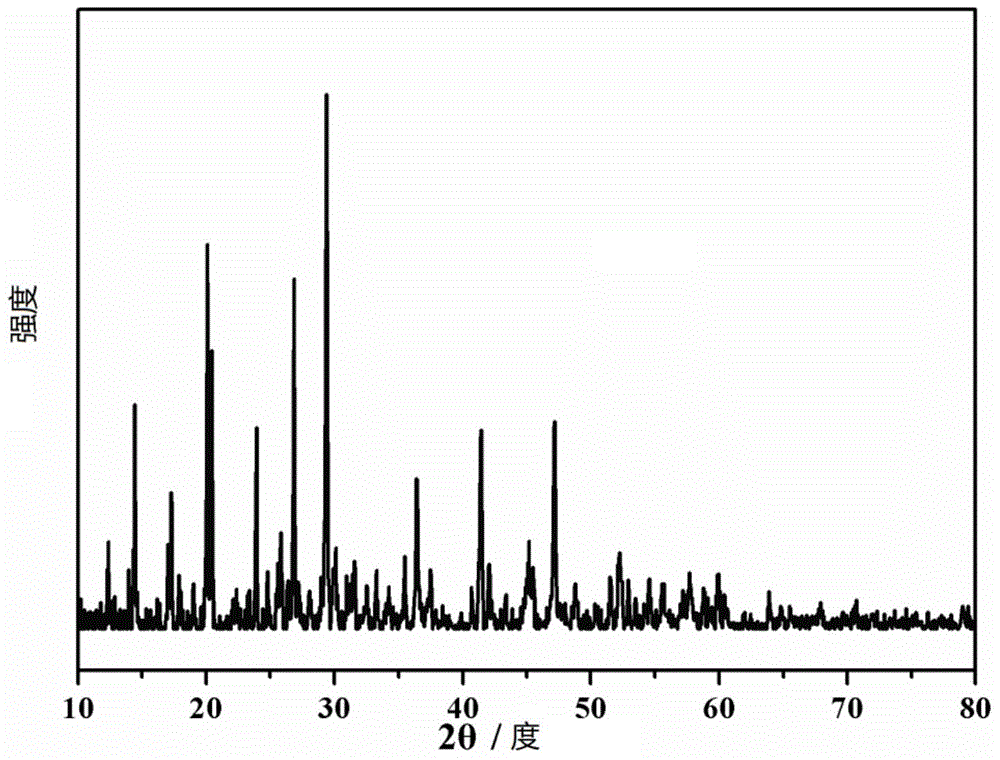
[0048] (1) Will Li 2 S, SnS 2 with P 2 S 5 According to the molar ratio of 5:1:1 in the high-energy ball milling device (the high-energy ball mill of Retsch company PM 400 model, the same below) under the argon atmosphere, the ball milling speed is 100rpm for 1h, until the mixture is uniform, and the powder obtained by mixing is placed under 10MPa The pressure is pressed into a sheet, and the obtained sheet is calcined at 600°C for 8 hours in an argon atmosphere. The solid material obtained by XRD is a crystalline inorganic solid electrolyte, which is composed of Li 10 SnP 2 S 12 crystal particles, the XRD pattern is shown in figure 2 as shown;
[0049] (2) According to the mass ratio of 4:1, the obtained Li 10 SnP 2 S 12 Crystal particles and Li 2 S and P 2 S 5 mixture (Li 2 S and P 2 S 5 Li in the mixture 2 S and P 2 S 5 The mola...
Embodiment 2
[0052] This example is used to illustrate the solid electrolyte material of the present invention and its preparation method.
[0053] According to the method described in embodiment 1, the difference is that in step (2) Li 2 S and P 2 S 5 Li in the mixture 2 S and P 2 S 5 The molar ratio of is 80:20, according to the weight ratio 7:3 will gain Li 10 SnP 2 S 12 Crystal particles and Li 2 S and P 2 S 5 The mixture is mixed, and it is measured by the method of transmission electron microscopy and electron diffraction analysis in Li 10 SnP 2 S 12 The in situ growth of crystal grains on the surface has a composition of 80Li 2 S-20P 2 S 5 amorphous inorganic solid electrolyte.
[0054] The resulting solid electrolyte material was pressed into tablets and tested, and its ionic conductivity at 25°C was 2.49×10 -3 S / cm, the ionic conductivity at 100°C is 8.26×10 -3 S / cm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ionic conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com