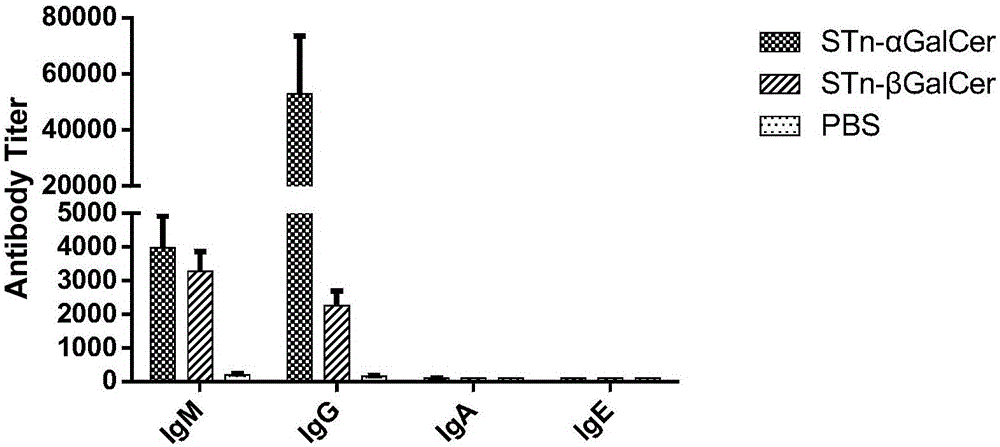
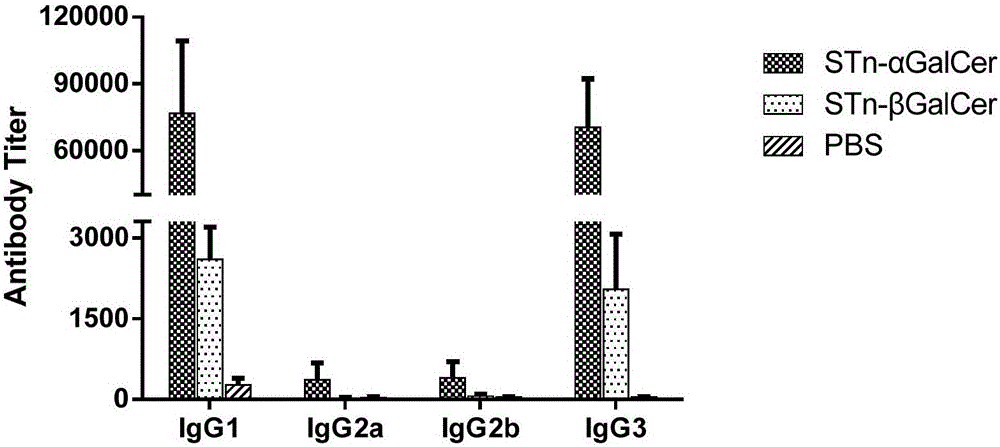
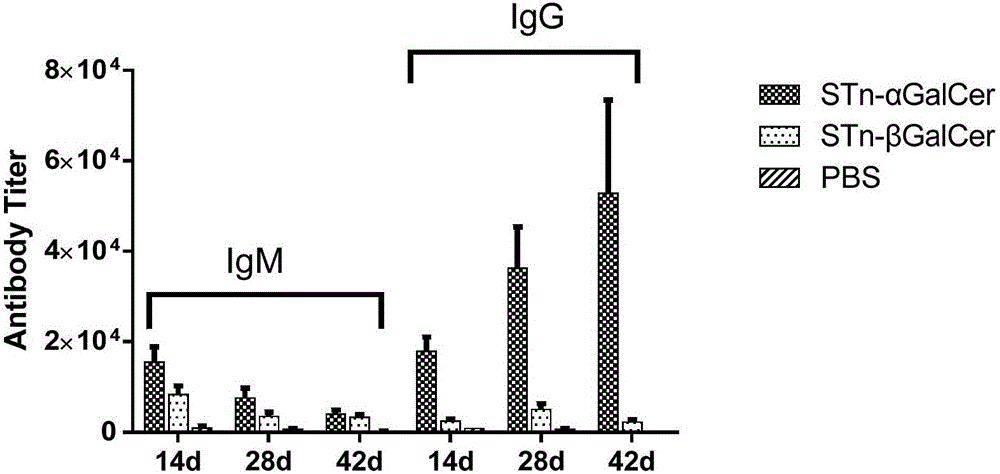
Liposomal vaccine preparation and use thereof, as well as method for producing IgG antibody
A liposome and preparation technology, applied in the field of liposome vaccines, can solve the problems of uncertain coupling sites, low efficiency, toxicity, etc., and achieve high affinity, low immune response, and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0095] According to a specific embodiment, when components (a), (b) and (c) are covalently connected, the vaccine molecule preferably has the following structure:
[0096]
[0097]
[0098] When the components (a), (b) and (c) are covalently connected, the molar ratio of the content of the components (a), (b) and (c) is 1:1:1.
[0099] According to another specific embodiment, the components (a) and (b) are covalently connected, and the connection products (component ab) and (c) are self-assembled by mixing, and the components ab and (c) The molar ratio of the content is 1:0.01-10, preferably 1:0.1-10.
[0100] The structures of the mixed component ab and component (c) are as follows: Among them, component (a) is STn, the right end of component (b) is βGalCer, and component (b) is βGalCer and covalently connected In the connection product of the connection unit in the mode, component (c) is selected from at least one of the illustrated structures.
[0101]
[0102] In the present inv...
Embodiment 1
[0115] This example is used to illustrate (a) the tumor-associated carbohydrate antigen STn (compound S1, STn antigen protected by a protecting group) and the covalent connection of the aforementioned carbohydrate antigen to the linking unit (compound 4).
[0116]
[0117] Compound 3 (200mg) was dissolved in dichloromethane / methanol (v / v 1:1), after adding palladium carbon, hydrogen gas was added, stirred for 1-2h, filtered through Celite, and the filtrate was decompressed to remove the solvent to obtain the product S1. Dissolve S1 in CH 3 CN, add p-nitrophenol adipate (compound 4, linking arm), react overnight at room temperature, and separate by silica gel column chromatography. The eluent ratio is dichloromethane / methanol=20:1 to obtain product 5 (180mg ) Colorless oily liquid. The above product 5 is the product of the covalent connection of the tumor-associated sugar antigen STn and the linking arm. The characterization data of product 5 are: 1 H NMR(400MHz, CDCl 3 )δ8.20(d...
Embodiment 2
[0119] This example is used to illustrate the adjuvants S9α, S10α, S11α, S12α and S13α for covalent attachment of the present invention and their preparation methods
[0120]
[0121] Add compounds S2 and S3 into the dry Add dry tetrahydrofuran to the molecular sieve reaction flask, stir for one hour and then cool to -20°C, add BF 3 ·Et 2 O solution, and kept at this temperature for 2h. After the reaction, it was diluted with ethyl acetate and filtered with Celite. Add saturated sodium bicarbonate solution to remove BF 3 ·Et 2 O, the product is separated by a silica gel column, the eluent ratio is petroleum ether / diethyl ether 4:1, and the products S4α and S4β are obtained.
[0122]
[0123] Dissolve S4α in a system with a volume ratio of acetic acid / water / tetrahydrofuran (v / v / v 8:2:5), stir at 60°C for 3-4 hours to obtain the product S5α, remove the solvent under reduced pressure, and add trimethyl The tetrahydrofuran solution of phosphorous was stirred for 3-5h to obtain the pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com