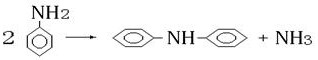
A kind of method that improves the synthetic diphenylamine conversion rate of aniline
A diphenylamine conversion rate, aniline technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry and other directions, can solve problems such as low conversion rate of diphenylamine, and achieve the improvement of aniline conversion rate, increase conversion rate, and increase economy. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]Preparation of the catalyst: The catalyst used in the experiment was a catalyst composed of β zeolite, activated alumina and alkali metals. The specific preparation method is as follows:
[0030](1) Take 200gHβ zeolite (SiO2 / Al2O3The molecular ratio is 28) and 1500 mL (0.1 mol / L) potassium chloride aqueous solution are placed in a 2000 mL four-neck flask with a stirrer for ion exchange. The exchange reaction temperature is 75°C, the stirring speed is 200n / min, and the time is 5.0h. Afterwards, the exchanged zeolite and potassium aqueous solution are filtered, washed, and dried; the washing process is: washing until there is no chlorine. The drying process is: drying at 60°C for 4.0 hours, and drying at 110°C for 4.0 hours.
[0031](2) Mix 70g of the above-mentioned exchanged zeolite with 10g of aluminum hydroxide powder, and add nitric acid and an appropriate amount of deionized water. The added amount of nitric acid accounts for 0.5wt% of the dry material. After kneading on an extr...
Embodiment 2~4
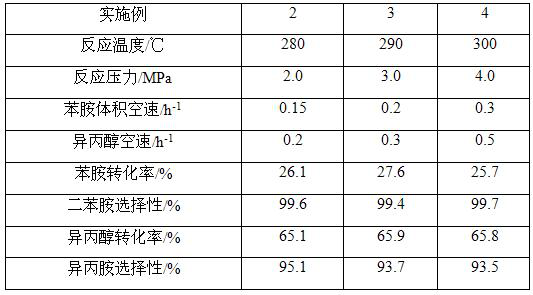
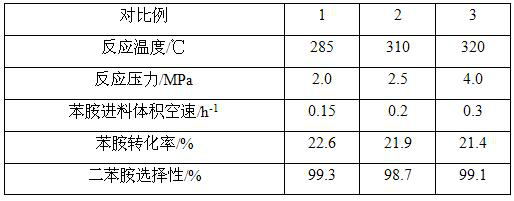
[0033]A fixed-bed reactor was used, the catalyst prepared in Example 1 was used, and 200 mL of the catalyst was taken and put into a stainless steel reactor with an inner diameter of 25 mm and a length of 1200 mm. The purity of the reaction raw material aniline>99%, purity of isopropanol>99%. The bottom feed method is adopted to pass aniline and isopropanol into the reactor at the same time. The synthesis reaction is carried out under different reaction temperature, pressure and feed space velocity. The reaction product flows out from the top of the reactor and enters the separator after cooling in. The composition was analyzed by gas chromatography, and the specific reaction conditions and results are shown in Table 1.
[0034]Table 1. Reaction conditions and results of Examples 2 to 4
[0035]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com