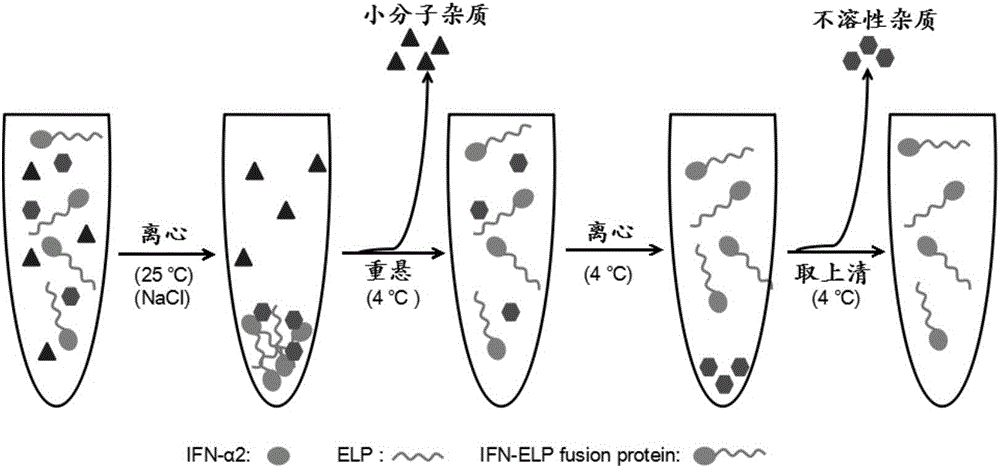
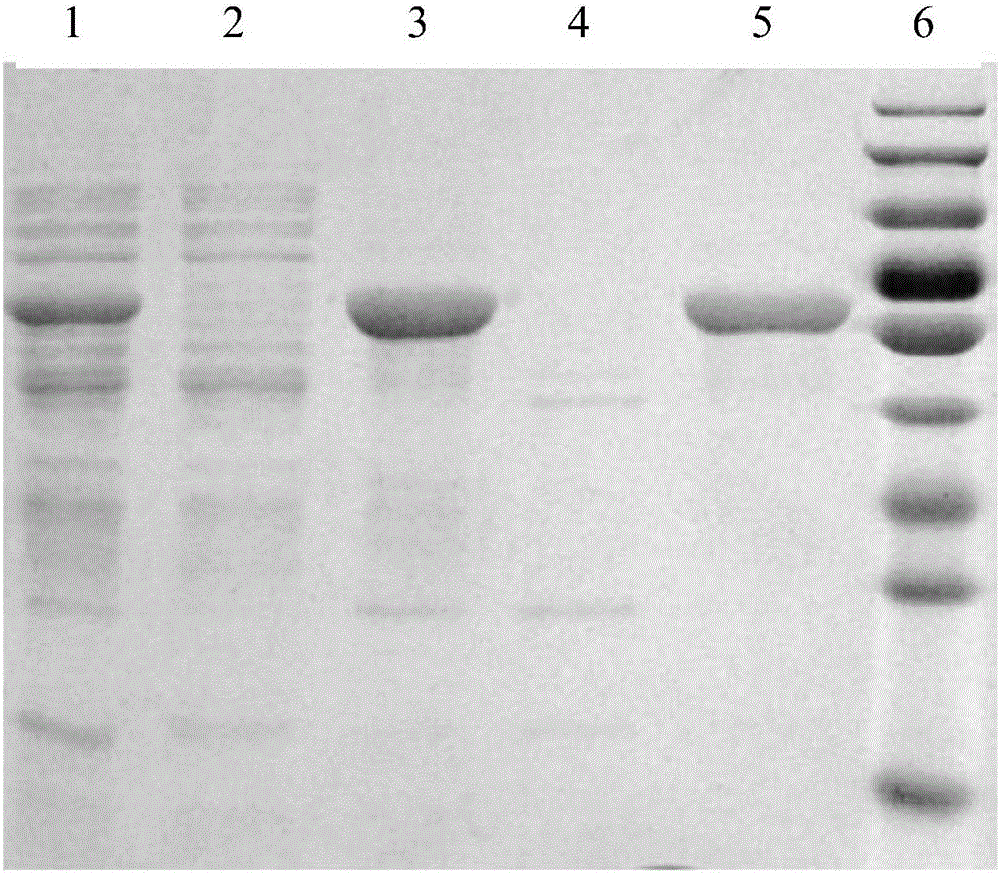
Fusion protein IFN-ELP and application thereof
A fusion protein and protein technology, applied in the field of biomedicine, can solve the problems of difficult control of binding sites and coupling stoichiometry, prolonging the half-life of interferon circulation, and ineffective clinical test effects, and achieves improved pharmacokinetics, in vivo and other problems. The effect of prolonging the average retention time and improving the stability of the drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Prokaryotic expression and purification of embodiment 1, IFN-ELP and transpeptidase Sortase
[0083] 1. Preparation of recombinant plasmid pET-25b-IFN-ELP
[0084] Artificially synthesized recombinant plasmid pET-25b-IFN-ELP (double-stranded circular plasmid). The nucleotide sequence of the recombinant plasmid pET-25b-IFN-ELP is shown in sequence 1 of the sequence listing. There is an expression cassette A in the recombinant plasmid pET-25b-IFN-ELP.
[0085] The nucleotide sequence of the expression cassette A is shown in the 5121 to 7314 positions from the 5' end of Sequence Listing Sequence 1, wherein the 5121 to 5140 positions from the 5' end are T7 promoters, and the 5209 to 5706 positions are interferon- In the coding gene of α2, positions 5728 to 5745 are the recognition region of transpeptidase A, positions 5812 to 7161 are genes encoding elastin-like polypeptide, and positions 7265 to 7314 are T7 terminators. Interferon-α2 is hereinafter referred to as IFN. ...
Embodiment 2
[0119] Obtaining of embodiment 2, IFN and ELP
[0120] The steps to obtain IFN and ELP are as follows:
[0121] 1. Take 10 mL of the 100 μM SrtA solution prepared in step 3 of Example 1, add CaCl 2 And make the concentration 10mM to obtain a solution.
[0122] 2. Take 10 mL of the 200 μM IFN-ELP solution prepared in step 3 of Example 1, add 5 mM triglycine (product of sigma company) to it, then mix it with 10 mL of the solution obtained in step 1, and react overnight at room temperature to obtain the mixed solution 1 . The mixed solution 1 was passed through a HiPrep 26 / 10 Desalting column (product of GE Company) with pH 7.4 and 10 mM PBS buffer to remove small molecular impurities to obtain a solution, and the mixed solution 2 was obtained.
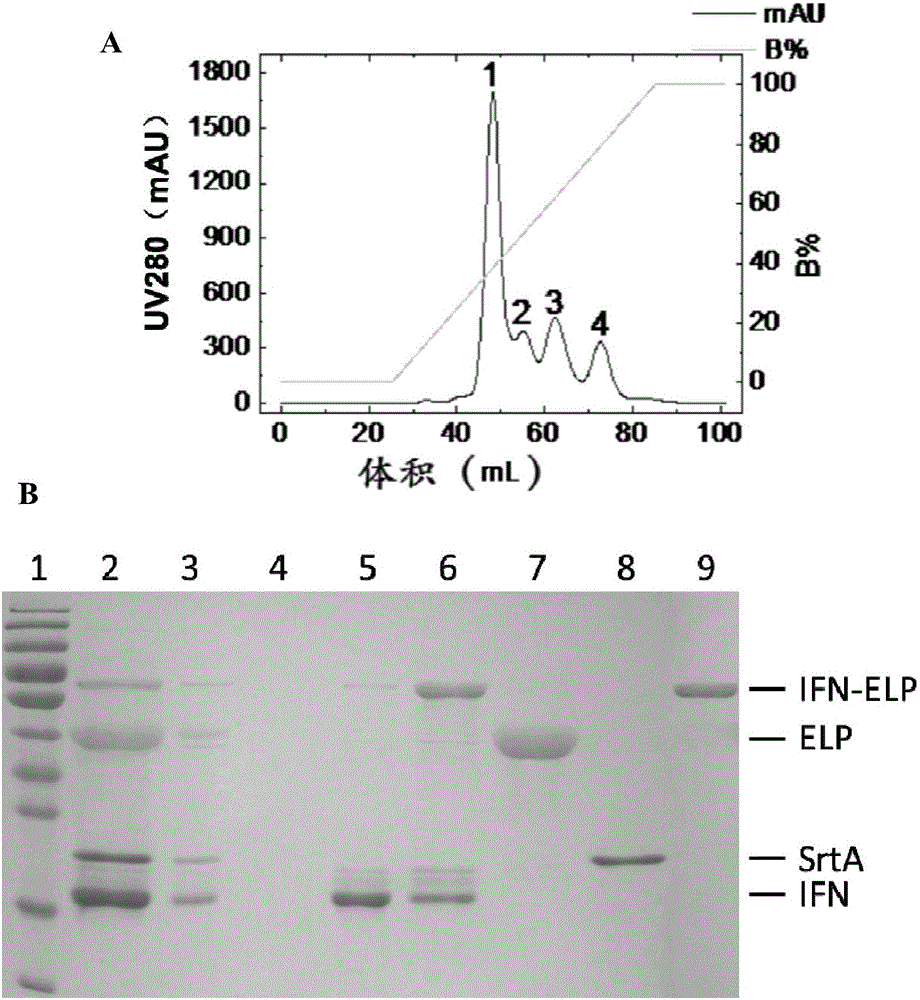
[0123] 3. After completing step 2, the mixed solution 2 was purified by anion exchange chromatography (HiTrap Capto Q 5 mL) on an AKTA protein purification system (AKTA Purifier 10, product of GE Company) to obtain an ELP solution.
...
Embodiment 3
[0128] The physicochemical characterization of embodiment 3, IFN-ELP
[0129] 1. Determination of molecular weight
[0130] The molecular weights of IFN-ELP, IFN and ELP obtained in the above steps were determined by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF), and the instrument was 4800Plus MALDI-TOF / TOF TM Analyzer (AB SCIEX), for specific measurement methods and steps, please refer to the instruction manual that comes with the instrument.
[0131] the result shows( Figure 5 ), IFN, ELP and IFN-ELP were purified by SrtA catalysis and anion exchange column (AEX). The measured molecular weight of IFN-ELP was 58236.1, and that of IFN was 20338.1, both of which were basically consistent with the theoretical values.
[0132] 2. Hydration radius
[0133]Dissolve the protein to be tested (IFN-ELP or IFN) with pH 7.4, 10 mM PBS buffer, and then filter through a 0.22 μm pore size filter to obtain the sample to be tested. The samples...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Phase transition temperature | aaaaa | aaaaa |
| Phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com