Method for extracting lithium from salt lake brine by extraction method
A technology for extracting lithium from salt lake brine is applied in the field of lithium extraction, which can solve the problems affecting the extraction ability of regenerative organic phase lithium, high requirements for anti-corrosion of extraction equipment, and high overall production cost, and achieves water solubility and chemical degradation. Application, the effect of reducing the cost of the extraction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
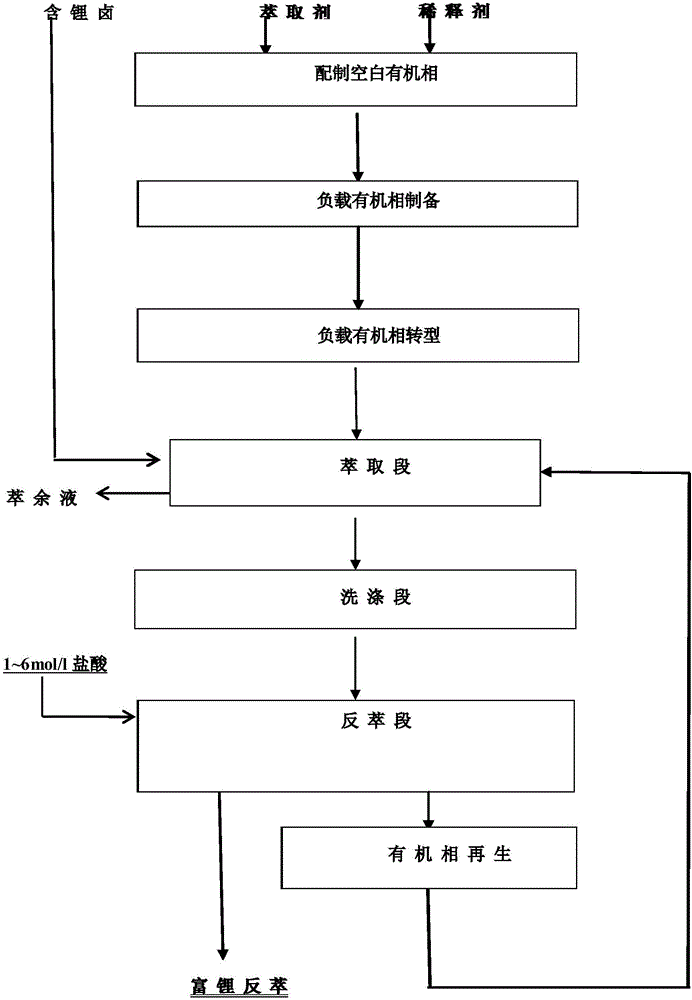
[0029]The neutral phosphorus oxygen compound A is tributyl phosphate TBP, the phase regulator B is the amine compound triisooctylamine N235 and the high-carbon alcohol compound sec-octanol, the diluent is sulfonated kerosene to form the extraction agent, tributyl phosphate The ester TBP accounts for 10%-40% by volume in the extractant.
[0030] Shake 1 part of extractant and 1 part of 0.1-6 mol / l hydrochloric acid solution with ferric chloride concentration of 0.1-3 mol / l for 10 minutes, then stand still for phase separation to obtain a loaded organic phase loaded with iron and acid; mix 1 part of the above-mentioned loaded Shake the organic phase with 4 parts of 3-6 mol / l calcium chloride solution for 10 minutes, then stand still and separate the phases to complete the transformation of the loaded organic phase; Lithium-containing brine (Mg / Li=100:1 mass ratio) was shaken for 10 minutes for extraction. After shaking, the phases were separated and the organic phase and aqueous...
Embodiment 2
[0032] Neutral phosphorus oxygen compound A uses trioctyl phosphate TOP, phase regulator B uses amine compound primary amine N1923 and higher carbon alcohol compound n-heptanol, diluent uses sulfonated kerosene to form extraction agent, trioctyl phosphate TOP It accounts for 10%-40% by volume in the extractant.
[0033] Shaking 1 part of extractant with 1 part of saturated magnesium chloride solution with ferric chloride concentration of 0.1 to 1 mol / l and hydrochloric acid concentration of 0.1 to 1 mol / l for 10 minutes and then standing for phase separation to obtain a loaded organic phase loaded with iron and acid; 1 part of the above-mentioned loaded organic phase and 6 parts of 3-6 mol / l magnesium chloride solution were shaken for 10 minutes and then left to separate the phases to complete the transformation of the loaded organic phase; Lithium-containing brine (Mg / Li=50:1 mass ratio) in a salt lake was shaken for 10 minutes for extraction. + , Mg 2+ 、Na + and K + Conc...
Embodiment 3
[0035] Neutral phosphorus and oxygen compound A uses tributyl phosphate TBP, phase regulator B uses amine compound primary amine N1923 and higher carbon alcohol compound secondary octanol, diluent uses sulfonated kerosene to form the extractant, tributyl phosphate TBP It accounts for 10%-40% by volume in the extractant.
[0036] Shaking 1 part of extractant with 1 part of saturated magnesium chloride solution with ferric chloride concentration of 0.1 to 1 mol / l and hydrochloric acid concentration of 0.1 to 1 mol / l for 10 minutes and then standing for phase separation to obtain a loaded organic phase loaded with iron and acid; 1 part of the above-mentioned loaded organic phase and 6 parts of 3-6 mol / l magnesium chloride solution were shaken for 10 minutes and then left to separate the phases to complete the transformation of the loaded organic phase; Lithium-containing brine (Mg / Li=50:1 mass ratio) in a salt lake was subjected to three-stage countercurrent extraction, and the o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com