Separation detection method of phenol compounds in textiles
A technology of phenolic compounds and detection methods, which is applied in the field of separation and detection of phenolic compounds in textiles, can solve the problems of time-consuming, inability to detect chlorophenol and o-phenylphenol at the same time, large amount of organic solvents, etc. Less dosage, overcoming time-consuming and polluting environment, good reproducible effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
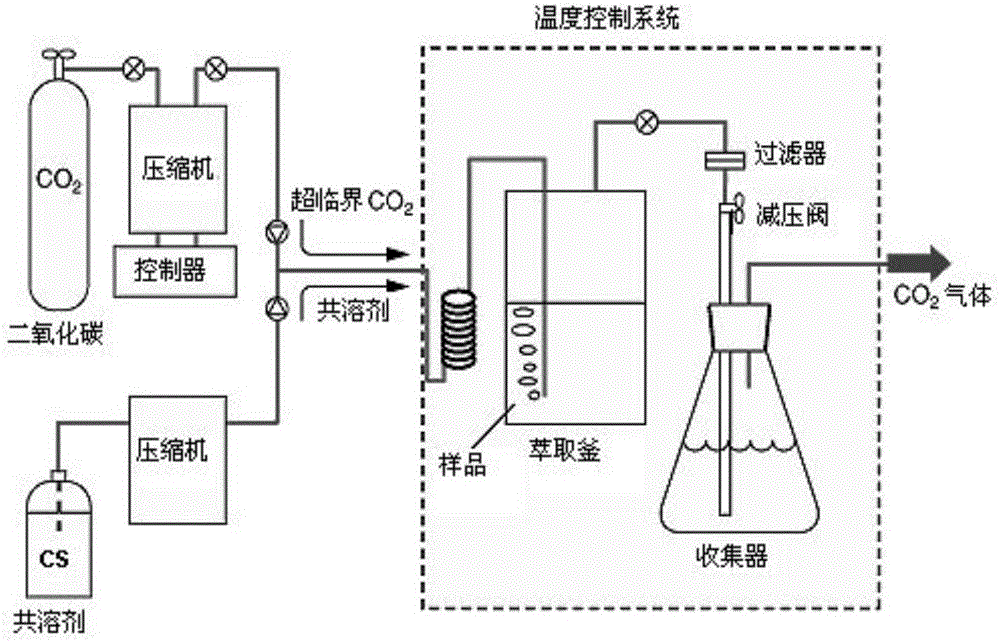
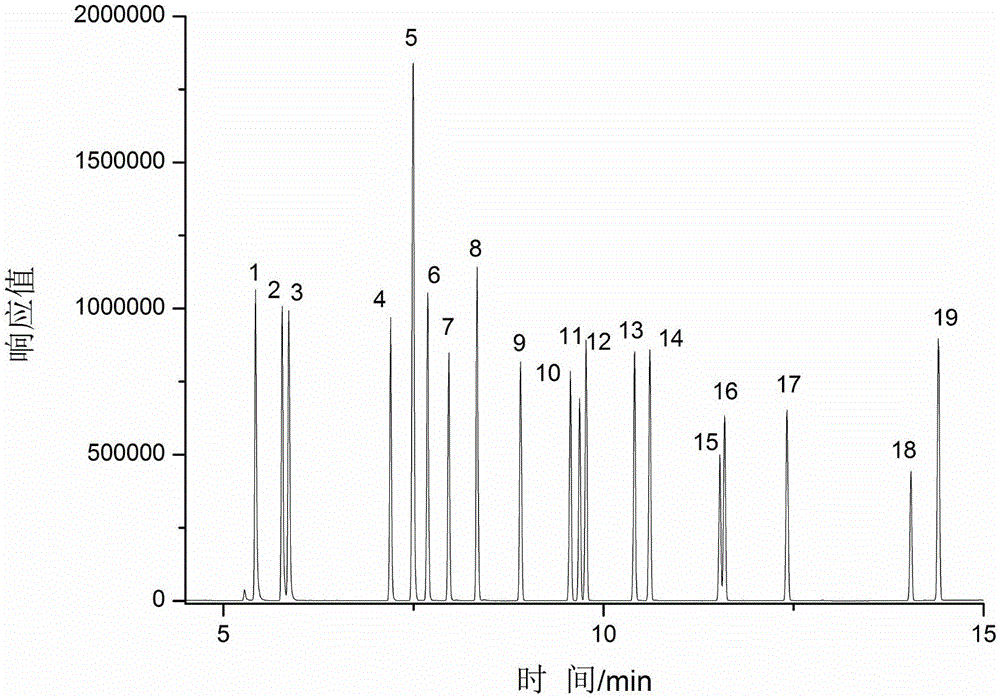
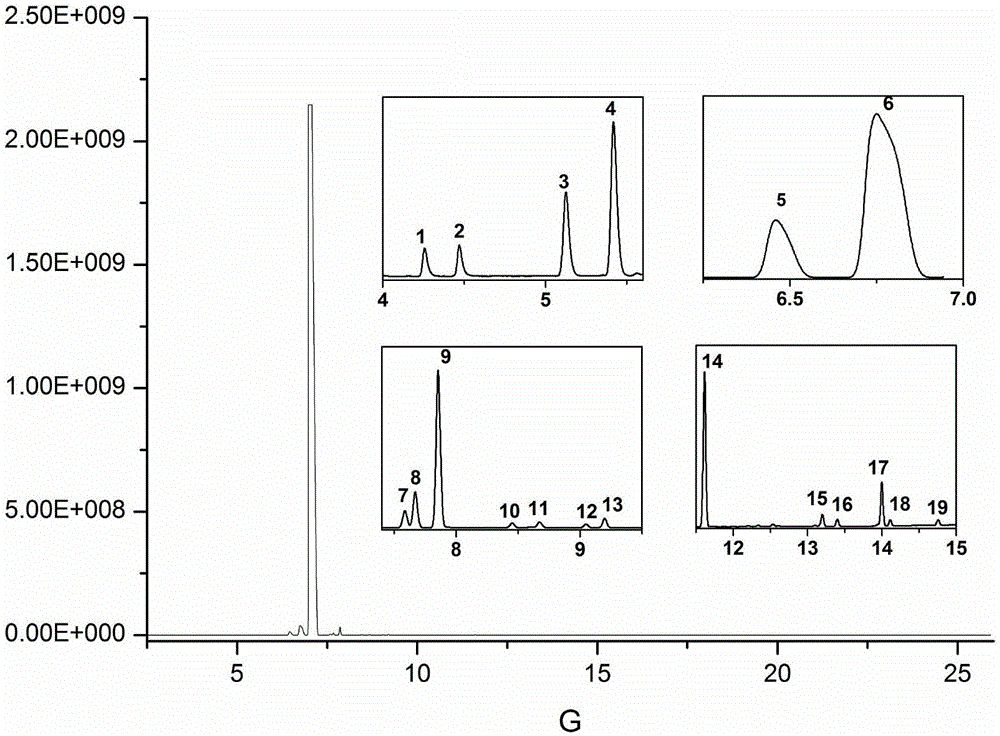
[0034] 10g textile sample is joined in the extraction kettle (supercritical carbon dioxide extraction flow chart sees figure 1 ), add 500 μL acetic anhydride and 500 μL co-solvent-methanol, set the extraction temperature to 50 ° C, the pressure to 35 MPa, fill with 30 mL of supercritical CO 2 The textile samples were subjected to static extraction with supercritical carbon dioxide for 10 minutes; then dynamic extraction for 15 minutes at the same temperature and 2 The flow rate is 2.0mL / min; the discharge valve is opened, and the extracted target substance is dissolved in 15mL of n-hexane in a cooled separation collector, and 3mL of potassium carbonate solution with a mass fraction of 1% is added to the extract, shaken for 1min, and n-hexane The alkane phase was dehydrated with 1 g of anhydrous sodium sulfate to obtain the sample solution to be tested; the sample solution to be tested was analyzed qualitatively and quantitatively by gas chromatography-mass spectrometry (GC-MS)...
Embodiment 2
[0042] Add 10g of textile samples into the extraction kettle, add 500μL of acetic anhydride and 500μL of co-solvent-dichloromethane, set the extraction temperature to 50°C, the pressure to 40MPa, and fill with 30mL of supercritical CO 2 The textile samples were subjected to static extraction with supercritical carbon dioxide for 5 minutes; then dynamic extraction at the same temperature and pressure for 20 minutes, supercritical CO 2 The flow rate is 1.2mL / min; open the discharge valve, and the extracted target substance is dissolved in 15mL of toluene in a cooled separation collector, and 3mL of potassium carbonate solution with a mass fraction of 1% is added to the extract, shaken for 1min, and the toluene phase After dehydration with 1g of anhydrous sodium sulfate, the sample solution to be tested was obtained; the sample solution to be tested was analyzed qualitatively and quantitatively by GC-MS or GC-FID. The test conditions of GC-MS and GC-FID are the same as in Example...
Embodiment 3
[0044] Add 10g of textile samples into the extraction kettle, add 500 μL of acetic anhydride and 500 μL of co-solvent-acetone, set the extraction temperature to 70°C, the pressure to 30MPa, and the flow rate of supercritical carbon dioxide to 8.0mL / min, the textile samples are subjected to super Dynamic extraction with critical carbon dioxide for 30 min; add 15 mL of ethyl acetate to the collection device, open the discharge valve, collect the precipitate, add 3 mL of potassium carbonate solution with a mass fraction of 1% to the extract solution, shake for 1 min, and ethyl acetate phase 1g of anhydrous sodium sulfate was dehydrated to obtain the sample solution to be tested; the sample solution to be tested was analyzed qualitatively and quantitatively by GC-MS or GC-FID. The test conditions of GC-MS and GC-FID are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com