Preparation method of hydrocracking catalyst
A hydrocracking and catalyst technology, which is applied in the direction of physical/chemical process catalysts, molecular sieve catalysts, chemical instruments and methods, etc., can solve the problems affecting the efficient performance of acidic functions, catalyst coking deactivation, weak formation, etc., to achieve good resistance Ability to poison nitrogen compounds, improve hydrogenation activity, and protect unimpeded effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The preparation method of the hydrocracking catalyst provided by the present invention specifically comprises:
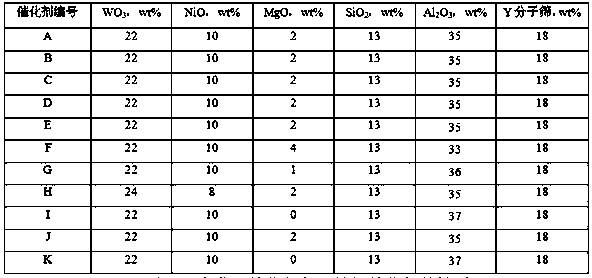
[0031] (1) Add alkaline solution B containing tungsten to acidic solution A containing magnesium, aluminum and silicon until the pH is 10-12 to obtain a mixed slurry;
[0032]Prepare magnesium, aluminum, and silicon-containing acidic solution A according to the content ratio of the catalyst components. The aluminum-containing salt can be one or more of aluminum chloride, aluminum nitrate, aluminum sulfate, etc., and the magnesium-containing salt can be magnesium chloride, nitric acid, etc. One or more of soluble magnesium salts such as magnesium and magnesium sulfate, the silicon source can be one or more of water glass, silica sol, etc.; the acidity can be one or more of inorganic or organic acids such as hydrochloric acid, nitric acid, and acetic acid One or more adjustments, it is advisable that the acidic salt solution is not turbid;
[0033] The auxilia...
Embodiment 1
[0046] Add 3000mL of clean water into a gelling tank and raise the temperature to 50°C;
[0047] Add 2000mL of clean water, 15mL of concentrated hydrochloric acid, 460g of aluminum chloride, 25g of magnesium chloride into a container, stir to dissolve, and drop in the 2 500mL of 131g / L dilute water glass solution, stir evenly, and prepare acidic solution A;
[0048] Prepare 1000mL of sodium hydroxide solution with a concentration of 15wt% in a container, add 25g of sodium tungstate, stir and dissolve, and prepare alkaline solution B;
[0049] Fix the flow rate of the acidic solution A, adjust the flow rate of the alkaline solution B, and flow into the gelation tank, control the gelation temperature at 50°C, the pH of the slurry at 10.8±0.2, and complete the gelation within 30 minutes. When the reaction is over, the A solution and B solution must all be added to the gel tank, and stirred for 10 minutes after the gel is completed;
[0050] (2) Add 3000L of clean water into a g...
Embodiment 2
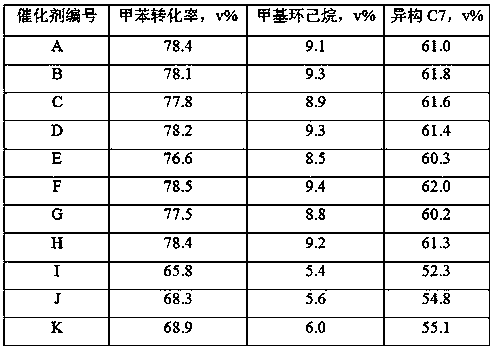
[0058] The whole preparation process of the catalyst is the same as in Example 1, the difference is that the catalyst preparation dosage is 1 / 5 of that of Example, 7 g of sodium tungstate is added when preparing alkaline solution B, and 22 g of sodium tungstate is added when preparing alkaline solution D, and mixed A part of molecular sieve slurry B1 in step (4) in Example 1 was added to the slurry to obtain catalyst B of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com